Updated requirements for fertilizer and supplement products that are, or contain, polymers
Products that are or contain polymers are themselves considered supplements and require registration before importation or sale in Canada. This change applies to stand-alone products and those used in combination with a fertilizer or supplement (for example, as a coating).
Previously, the definition of a "supplement" was limited to materials that had direct effects only. Under this former interpretation, polymers intended for use with fertilizers and supplements required a comprehensive safety assessment before entering the market, but did not need to be registered when sold or imported into Canada as stand-alone products.
Polymers are materials made of long, repeating chains of molecules. The materials have unique properties, depending on the type of monomers being bonded and how they are bonded. For a given polymer, the presence of unbound residual monomers, polymerisation chemicals, additives and impurities, and the type and quantity of degradation products can significantly affect the safety of the final product. Therefore it is important to ensure that fertilizers and supplements that are or contain polymers do not pose a risk of harm to humans, plants, animals or the environment through mandatory pre-market assessment and product registration.
Benefits to registering products that are or contain polymers:
- promote a safe and fair marketplace
- ensure that all polymeric supplements undergo a comprehensive safety assessment before importation and sale in Canada, minimizing any potential risk to human, plant or animal health or the environment
- ensure that polymeric supplements (sold either as stand-alone additives or mixed with exempt or registered ingredients) meet all the prescribed regulatory requirements, including labelling
- make the regulatory requirements clearer and more transparent
- align with the Government of Canada's commitment to protect Canadians and the environment from potential safety risks posed by various chemicals
Timeline
An upcoming Trade Memorandum will provide guidance on the regulatory requirements after the phased implementation period (April 1, 2021 – October 26, 2023).
The phased implementation and enforcement timeline is based on extensive consultation with the main industry associations in Canada, Fertilizer Canada and the Fertilizer and Supplement Advisory Committee. This approach allows time for polymer manufacturers to register their products and for down-stream producers and blenders to print amended labels and packaging using the newly issued registration numbers.
This implementation timeline is only applicable to products that are currently being sold in or imported into Canada after having undergone a comprehensive safety assessment by the CFIA. As long as no changes were made to active ingredients, their sources, the manufacturing process or intended end use pattern (non-food vs. food crops), the results of the existing safety assessment will be considered valid and no new or additional data will be required to support registration of the product under the Fertilizers Act.
These registrations will be processed in accordance with the service delivery standards for minor amendments, which has a maximum review timeline of 75 working days. (See Trade memorandum T-4-122 for more information on CFIA service standards for registration related applications.)
This process will allow polymeric supplement manufacturers to quickly transition their products to a registered status (including printing marketplace labels that are compliant with the Fertilizers Regulations), with the majority of the implementation period remaining for downstream producers and blenders to adjust their marketplace products and bring them into compliance by the deadline of October 26, 2023.
To promote transparency and awareness within the sector, it is recommended that polymer manufacturers inform their customers and provide confirmation once they have made a submission for registration to the CFIA so that customers have the documentation for themselves and to assist them in responding to enquiries about their distribution chain.
All other polymeric supplements (those in commerce that have not undergone a safety assessment or are new to the Canadian marketplace) must be registered before they can be legally sold or imported into Canada.
Further information
The information below outlines the requirements for polymer manufacturers and downstream producers starting from April 1, 2021 to October 26, 2023, as well as the actions the CFIA will take depending on a given scenario.
-
Polymeric supplements that have undergone a comprehensive safety assessment in the past and no changes have been made to the active ingredients, their sources, the manufacturing process or intended end use pattern (non-food vs. food crops) since that time.
Polymer manufacturers
- Submit the registration package to the CFIA by September 30, 2021.
- Inform your customers (downstream producers that use your product) of the status.
Downstream producers
- Prepare new labels in compliance with the amended Fertilizers Regulations – begin printing new labels once a valid registration number is obtained (January 1, 2022).
CFIA
- Process the registrations in accordance with the service delivery standard for minor amendments (up to 75 days). Consider the safety assessments conducted in the past to be valid (no new safety data required) and don't charge safety review fees.
- Conduct a marketplace survey to better gauge the size of the sector including the number and type of polymer products and technologies currently in commerce.
- Issue information letters to individual companies selling unregistered products that are or contain polymers.
- Defer enforcement actions until October 26, 2023.
-
Polymeric supplements that have undergone a safety assessment by the CFIA in the past but, since that time, changes were made to the active ingredients, their sources, the manufacturing process or intended end use pattern (non-food vs. food crops).
Polymer manufacturers
- Making substantive changes to the product after the safety assessment was completed puts the outcomes of assessment into question and the CFIA should have been informed of the changes prior to importation and sale. The product must be registered under the Fertilizers Act before any further importation or sale.
Downstream producers
- Exhaust old product stock throughout the distribution chain. Obtain valid registration number to print new labels.
CFIA
- Process registration applications in accordance with the service delivery standards for new registrations (up to 380 days). Conduct comprehensive safety assessment (level III) and charge safety fees. Defer enforcement against products in the retail chain until October 26, 2023 as long as an application for registration is under review by the CFIA.
-
The polymeric supplement was sold and imported into Canada without a valid safety assessment from the CFIA.
Polymer manufacturers
- The product is non-compliant with the Fertilizers Act and Regulations and should not have been sold or imported. It must be registered under the Fertilizers Act before any further importation or sale.
Downstream producers
- Exhaust old product stock throughout the distribution chain. Obtain valid registration number to print new labels.
CFIA
- Process registration applications in accordance with the service delivery standards for new registrations (up to 380 days). Conduct comprehensive safety assessment (level III) and charge safety fees. Enforcement action will be taken against these products when found unregistered in the marketplace.
-
Polymeric supplements that are new and have not reached the Canadian market.
Polymer manufacturers
- Submit for and obtain registration under the Fertilizers Act before the product is imported or sold in Canada.
Downstream producers
- Require registration numbers to be provided by your polymer supplier before using it in your blends, on seed or in growing media.
CFIA
- Process registration applications in accordance with the standard service delivery standards for new registrations (up to 380 days). Conduct comprehensive safety assessment (level III) and charge safety fees. Enforcement action will be taken against these products when found unregistered in the marketplace.
Click on image for larger view
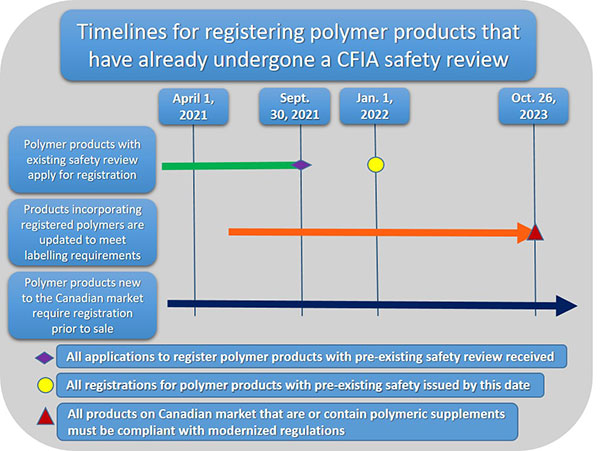
Description for timelines for registering polymer products that have already undergone a CFIA safety review.
This image describes the timelines for registering polymer products that have already undergone a CFIA safety review.
Polymer products with an existing safety review can apply from registration between now and September 30, 2021. All registrations will be issued by January 1, 2022.
Products incorporating registered polymers are updated to meet labelling requirements as polymers are registered and registration numbers are available to add to marketplace labels. All labels must be compliant by October 26, 2023.
Polymer products that are new to the Canadian market require registration prior to sale.
Applying for registration
When preparing an application for registration under the Fertilizers Act, polymers that are active ingredients must be guaranteed on the marketplace label as a minimum percentage of the final product formulation by weight. The description of the polymer must match the corresponding Chemical Abstract Service Registry Number (CAS RN) listing.
There are no new safety data requirements specific to polymers or products containing polymers; the CFIA uses the same risk assessment end points as for any other product requiring registration under the Fertilizers Act. These include:
- Toxicological hazard characterization:
- for the polymer(s)
- for other active and inert ingredient(s) and
- for the residual monomers, cross-linkers, catalysts, and any potential degradation products (where applicable)
- Process of manufacturing or extraction:
- relative proportions of monomer(s), cross-linker(s), and catalysts used in the manufacturing process
- analytical results to demonstrate the residual levels of these compounds in the final product
- Food-safety assessment:
- a scientific rationale and/or data to address the risk of uptake and incorporation of monomers, cross-linkers and any degradation products in the edible portion of food crops
- where applicable, existing upper tolerances for foods (for example, acrylamide) must be addressed by inclusion in the rationale or risk assessment
Notes:
If the polymer, any of its ingredients or degradation products meet the hazard criteria outlined in Appendix 4 of the Guide to Submitting Applications for Registration Under the Fertilizers Act; or are recognized to exhibit:
- (potential) carcinogenicity
- mutagenicity
- reproductive toxicity
- developmental toxicity
- teratogenicity or endocrine disruption activity
An exposure assessment for the intended use must be provided. The exposure assessment should also include mitigating factors, such as recommended personal protective equipment and precautionary statements.
If the product containing a polymeric supplement that is not intended for use on food-crops, or if food safety is not adequately substantiated, the statement, "not for use on food crops" must appear prominently on the marketplace label.
Mixtures containing polymeric supplements
Mixtures containing polymeric supplements are exempt from registration if all active ingredients in the mixture are either registered for the proposed use of the mixture or are exempt from registration (for example materials on the List of Primary Fertilizer and Supplement Materials).
If:
- the directions for use of a registered product included in a mixture are not consistent with the proposed use of the mixture, the final mixed product must be registered
- a mixture contains a product that requires registration but is not registered, then the final mixed product requires registration
Record keeping vs. labelling
Information on labelling and record keeping can be found in:
- T-4-130 Labeling requirements for fertilizers and supplements.
- T-4-131: record keeping requirements under the Fertilizers Act and Regulations.
- Date modified: