This page has been archived
Information identified as archived is provided for reference, research or record-keeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
February 1, 2016 – April 8, 2016
Table of contents
- Introduction
- What we heard
- Written feedback
- Respondent profile
- Key respondent messages
- Scope of the proposed framework
- Modernized regulatory requirements
- Permissions – General comments
- Permissions – Ingredients
- Permissions – Mixed feeds
- Permissions – Persons or establishments
- Permissions – Persons or establishments (domestic)
- Permissions – Persons or establishments (imports)
- Permissions – Persons or establishments (exports)
- Next steps
Introduction
The Canadian Food Inspection Agency (CFIA) has embarked on a comprehensive change agenda to strengthen its foundation of legislation, regulatory programs and inspection delivery. These directions set the context for renewing the Feeds Regulations (Regulations).
The goal of modernizing the Regulations is to reduce compliance burden and support innovation while maintaining animal and human health, as well as environmental and economic stability. The modernization of the Regulations is designed to benefit the collective Canadian feed industry, which includes livestock producers, commercial feed manufacturers, retailers, importers, exporters, ingredient manufacturers and food processors. In addition to aligning with other international feed regulatory regimes, modernization also aims to enhance animal health and food safety for the Canadian public.
As a next step in its regulatory development process, the CFIA prepared the Feed Regulatory Renewal Consolidated Modernized Framework Proposal ("Consolidated Proposal") for further consultation with stakeholders. The proposal sought to:
- Integrate the first three modules (Feed Ingredient Assessment & Authorization, Feed Labelling and Feed Hazard ID and Preventive Controls) to demonstrate how they will work together to provide a robust, risk-based regulatory framework;
- Add information on facility permissions (licensing/inspection/enforcement) to demonstrate how the principles of the modernized Integrated Agency Inspection Model (iAIM) will apply in a feed context; and
- Propose additional key regulatory requirements not addressed in the proposals/consultations to date.
Figure 1 illustrates the scope of the proposed modernized regulatory framework (key regulatory requirements and permissions), which will apply to domestic regulated parties, imports and exports. The Consolidated Proposal discussed each of the aspects in the illustration in detail, and stakeholders were prompted to comment on the aspects most important to them.
This report consolidates and summarizes the comments received during the consultation period and the CFIA's response to those comments.
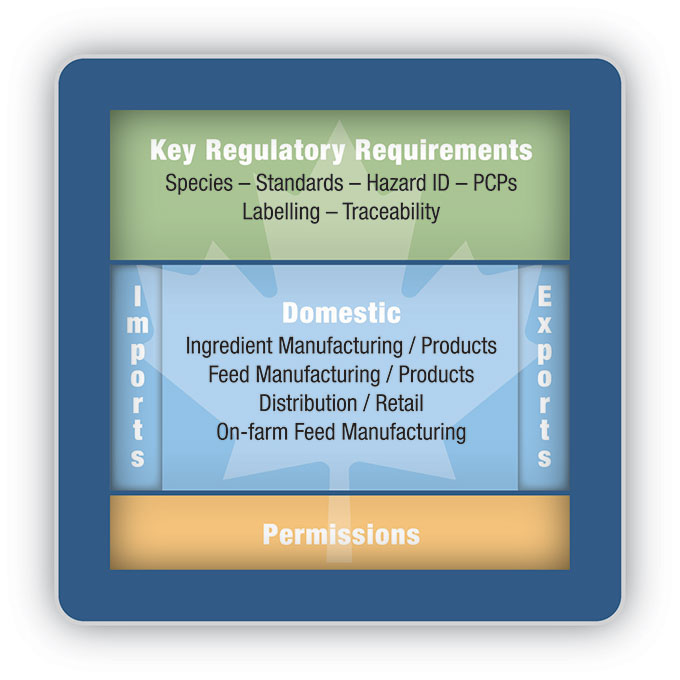
Description for Figure 1
"Resembling a tablet computer, the graphic identifies the 2 main groupings of requirements of the proposed modernized framework with the headings "Key Regulatory Requirements", highlighted in a green band across the top of the tablet's "screen" and "Permissions", highlighted in an orange band across the bottom. In the middle of the "screen", from left to right, the headings, "Import", "Domestic" and "Export" are highlighted in a blue band. Under the "Domestic" heading in the middle, "Ingredient Manufacturing / Products", "Feed Manufacturing / Products", "Distribution / Retail" and "On-farm Feed Manufacturing" are listed. The graphic shows that the Key Regulatory Requirements and Permissions will apply to all 3 levels of trade (import, export and domestic)
About the consultation
The Agency used three principal mechanisms to advance the consultation:
- The staging of eight (8) public "town hall" meetings across Canada during February-March 2016;
- The staging of three webinars to inform and invite feedback from CFIA staff; and
- The distribution and posting of the Consolidated Proposal on the CFIA website.
The Agency also provided a 60+ day consultation period on the Consolidated Proposal, soliciting comments from interested parties from February 1, 2016, to April 8, 2016.
The CFIA would like to thank everyone who participated in the consultation opportunities that were provided, for contributing their time to the consultation process and sharing their views.
What we heard
Town hall meetings
To further engage Canadians and a broader range of stakeholders and interested parties, the CFIA staged a series of public "town hall" sessions across Canada to provide information to more regionally- and provincially-based stakeholder and partner organizations, and to encourage their feedback on the consolidated framework consultation proposal prepared by the CFIA.
During February and March of 2016, eight (8) town halls were staged across Canada (Moncton, NB; Saint-Hyacinthe, QC; Guelph, ON; Ottawa, ON; Winnipeg, MB; Saskatoon, SK; Edmonton, AB, and Abbotsford, BC).
Response to the regional town hall meetings was very positive, with 286 attendees in total. We heard the following comments from participants at many of these meetings:
- Stakeholders were generally pleased by the scope and direction of the modernized regulatory framework being proposed;
- Concerns were expressed regarding:
- the CFIA's intention to take a more transparent approach to assessing and approving feed ingredients,
- requirements to provide health and safety-related information on feed labels in both official languages;
- the exemptions being provided to livestock producers who manufacture feeds under the modernized Feeds Act, and
- domestic permissions to be required by persons or establishments doing business across provincial boundaries only.
| Category of participant | Distribution |
|---|---|
| Commercial feed industry – Association/Individual | 120 |
| Ingredient suppliers – Association/Individual | 74 |
| Livestock producer – Association/Individual | 41 |
| Other feed inputs | 8 |
| Government (Canadian federal/provincial) | 21 |
| Government (CFIA) | 22 |
| Total | 286 |
CFIA employee webinars
To further engage a broader range of CFIA staff in the consultation, the Animal Feed Division hosted three (3) webinars in March 2016 to provide information to nationally and regionally-based staff, and to encourage their feedback on the proposed consolidated framework. Some 80 employees registered for the three sessions in total. For the most part, these sessions provided CFIA staff the opportunity to ask questions or seek clarification on various aspects of the proposed framework.
Written feedback
Respondent profile
As illustrated in Table 2, the Agency received sixty-one (61) sets of written comments on the Consolidated Proposal from a range of respondents.
| Category of Respondent | Distribution |
|---|---|
| Commercial feed industry – Association | 5 |
| Commercial feed industry – Individual | 21 |
| Ingredient suppliers – Association | 4 |
| Ingredient suppliers – Individual | 6 |
| Livestock producer – Association | 6 |
| Livestock producer – Individual | 2 |
| Other feed inputs | 4 |
| Government (Canadian federal/provincial) | 2 |
| Government (CFIA) | 11 |
| Total | 61 |
The commercial feed industry association comments represent Canadian and American commercial feed manufacturers, as well as some of the larger feed ingredient groups. The Canadian commercial feed industry association represents 90 percent of commercial feed manufactured in Canada, while the US association represents about 75 percent of commercial feed manufactured in the US.
Key respondent messages
- Ingredient suppliers frequently commented on the aspect of the proposal that would require feed exporters to obtain a permission from the CFIA, with the majority of those who commented not in favour with this proposed scheme. The reasons provided for this position included:
- a permission would impose additional administrative burden on regulated parties;
- a permission would be redundant where a feed:
- would have to be manufactured in accordance with a preventive control plan;
- is subject to mandatory CFIA registration; or
- meets the importing country's requirements.
- a permission for exporters should be provided on a voluntary basis where export certification would be required by a trading partner.
- The commercial feed industry associations commented that the requirements in the proposal will not reduce the compliance burden, and will be costly for the industry to implement. The general comments from the other respondents were split between a general agreement with the proposal and concerns about compliance burden.
- Very few comments were received from livestock producers, either from organizations or individuals. These were concerned in particular with the proposal to expand the scope of species to which the modernized framework would be applied. While these groups represent a large proportion of purchasers of feeds, and the regulatory framework will impact some that import feeds or manufacture feeds on-farm, the limited comments that the CFIA did receive focused mostly on:
- maintaining the exemptions provided in the Regulations for on-farm feed manufacturing;
- concerns regarding the role third party safety and quality programs, already established within their sectors, will play within the context of meeting the CFIA's proposed preventive control plan (PCP) requirements; and
- concerns regarding the delays for new products to enter the domestic marketplace.
Many of the comments received by the CFIA in response to the Consolidated Proposal pertained to specific sections or requirements for feeds in the proposal, and sought clarification on particular points. A summary of, and the CFIA's responses to, the more specific respondent comments are set out below.
Scope of the proposed framework
In the Scope section of the Consolidated Proposal, the CFIA outlined those activities that would be subject to a risk and outcome-based feed regulatory framework, as well as those aspects that would not be regulated by the CFIA. Respondents raised concerns in response to this section of the proposal with regards to the exemptions from the Feeds Act and Regulations (including the changes proposed in the Consolidated Proposal) that would apply to livestock producers manufacturing feed that is not offered for sale, or has not had incorporated into it any drug or other substance that may pose a risk to human or animal health or the environment. They indicated that all those involved in the food chain and feed production system, including livestock producers manufacturing feed for their own animals, should be subject to the proposed requirements to maintain a consistent and fair approach to regulating livestock feeds in Canada, and to ensure protection for food safety, human and animal health, and the environment. A few respondents representing livestock producers were generally in agreement with the exemption provided in the Feeds Act and Regulations for livestock producers manufacturing feeds for their own animals, and suggested no changes were necessary.
Additional comments received suggested that transporters of livestock feeds should also fall within the scope of the modernized regulations and subject to the proposed requirements. The respondents noted that the transportation of livestock feeds has the potential of being a source of contamination, if adequate preventive controls are not implemented.
CFIA response:
While the CFIA appreciates the perspectives raised regarding the exemption provided for livestock producers manufacturing feeds for their own animals, these exemptions were largely carried over from the existing Feeds Act, and were specified within the text of the Agricultural Growth Act passed by the Parliament of Canada in 2015. As such, altering these exemptions falls outside the scope of the regulatory renewal project.
While the CFIA currently has no immediate plans to consult with the Canadian transportation sector concerning the application of the Feeds Act and Regulations to their industry, a feed establishment's preventive control plan will be expected to account for all applicable hazards, which may include those introduced via transportation of ingredients and finished feeds.
Modernized regulatory requirements
Species
This section introduced a modernized scope for the species to which the Regulations would apply, and proposed the removal of some non-food producing species. Respondents were generally in agreement with the proposal to expand the list of regulated species to include feeds for animals raised for human consumption, and exclude feeds for animals that do not enter the food or feed chain (i.e., mink and fox).
Additional comments suggested a more general approach to the list of regulated species by replacing the specific list with a more all-encompassing, outcome-based expression such as "feeds for species raised for human consumption".
There were also a couple of respondents who indicated their opposition to the proposed removal of mink and fox from the list of regulated species, stating that consumer protection for these groups may be equally important.
CFIA Response:
The CFIA agrees with the respondents that the proposed list of regulated species adequately captures those livestock currently raised for the purpose of human consumption.
The CFIA will propose a definition for "livestock" in the modernized Regulations to be published in the Canada Gazette Part I, and will seek to align the species for which feeds are regulated with those species that will be considered "food animals" in the Safe Food for Canadians Regulations.
The CFIA has considered incorporating a list of species by reference in the Regulations as an alternative approach to providing a definition in the Regulations themselves. However, it was concluded that the potential impacts on both regulated parties and government on making changes to the scope of species would warrant a more formal and rigorous regulatory change process than an administrative one. For a more detailed discussion on incorporation of documents by reference, please refer to the CFIA's Incorporation by Reference Policy.
After consultation with the mink and fox industries, it is considered appropriate for the CFIA to remove the oversight of their feeds from the scope of the regulations, as mink and fox are raised for their fur, and their products or by-products do not enter the food or feed chain.
Safety standards
This section proposed the establishment of maximum nutrient and contaminant levels, as well as re-emphasizing the principles of hazard identification and preventive control plans (PCP) that have been outlined in a previous consultation. The majority of respondents were in agreement with the proposed removal of Table 4 of Schedule I of the Regulations,indicating that the current nutrient guarantee standards are outdated and restrictive.
Most respondents also agreed that setting maximum levels/standards for some nutrients and contaminants would be an effective measure to ensure the safety of livestock feeds. However, concerns were raised regarding who would be responsible for developing these levels/standards. Respondents indicated the necessity that any standards established should be based on sound, current scientific evidence and involve consultation between the CFIA and stakeholders. Furthermore, specific concerns were raised by some respondents over setting maximum mycotoxin standards, citing that there is currently insufficient scientific literature for establishing critical limits.
Additional comments from respondents included:
- suggested amendments to the preventive control plan requirements;
- the preparation of industry tools/guidance materials with respect to hazard analysis; and,
- a request that the CFIA be mindful of the costs associated with testing for hazards in feeds.
CFIA Response:
The CFIA agrees that science-based determinations of maximum standards are required for an effective regulatory framework, and is committed to working with regulated parties and government partners to develop and maintain the list of maximum nutrient and contaminant standards. Proposals for such standards will be provided for stakeholder review and feedback prior to the pre-publication of the modernized regulations in the Canada Gazette.
Furthermore, the CFIA is committed to exploring opportunities for training and developing guidance materials for industry and CFIA inspection staff.
Labelling and standards of identity
In the Consolidated Proposal, the CFIA specified a number of strategies with respect to labelling requirements for feeds to provide additional flexibility while adding safety and traceability elements.
Complete list of ingredients
Most respondents were opposed to including a complete list of ingredients on feed labels, indicating that this requirement would:
- increase administrative burden when creating labels;
- disclose proprietary information to competitors; and,
- result in large, complex labels.
Several respondents felt that the current measure of allowing a purchaser to obtain the ingredient details from the manufacturer upon request achieves the objective of making information about feed composition available, and that this measure should not change. In addition, while there was no clear consensus with respect to the flexibility that collective terms for groupings of ingredients could provide, respondents indicated that if providing a complete list of ingredients was to be a requirement in the modernized regulations, allowing collective terms may help reduce some of the labelling burden. Additional collective terms would be necessary, however, to align Canadian collective terms and labels more closely with the collective terms permitted in the US (for example, a collective term for "animal protein products").
A few respondents were in favour of requiring a complete list of ingredients on feed labels, as they felt this would provide the purchaser with the knowledge required to make informed choices when purchasing their feeds.
CFIA Response:
The list of ingredients provides useful information to the purchaser of a feed, and will be more meaningful, as the number of feeds requiring mandatory registration will be limited. This requirement is consistent with international guidance provided by the Codex Alimentarius Code of Practice on Good Animal Feeding, federal feed labelling requirements administered by the USFDA and other international jurisdictions. The CFIA proposes the requirement to provide a complete list of ingredients to the purchaser at time of sale will continue, however, the CFIA is investigating alternative options with respect to how the list of ingredients must be provided.
As indicated above, there was no clear consensus with respect to the collective terms proposed by the CFIA (see Annex I – Revised Collective Terms of the Feed Labelling Collective Ingredient Terms Proposal Consultation Summary) and the flexibility they could provide in the labelling of feeds. Given the limited feedback provided along with the fact that the CFIA will be investigating alternative measures for providing ingredient information to the purchaser, the CFIA has concluded that it will not pursue the completion of a list of collective terms for incorporation into the regulations by reference as an option for reducing labelling burden.
Ingredient statements/Name and address
Respondents expressed concern regarding the transfer of caution or warning statements required on feed ingredient labels to labels of other feeds containing those ingredients, noting this would increase the labelling burden for industry and complicate the label information for the purchaser. Furthermore, respondents questioned whether the ingredient cautions or warnings were applicable once the ingredient has been diluted in a mixed feed.
Respondents were opposed to the proposal to require the manufacturer's name and the actual location of manufacture on feed labels as this would:
- release confidential information;
- create additional labelling burden for the feed industry; and
- create a disconnect with the US requirements of labelling the name and address of the manufacturer, distributor or packager.
CFIA Response:
The transfer of health and safety focused cautions and warnings for feeds containing such single ingredients will aid in the safe use of livestock feeds in Canada. The CFIA will further clarify which ingredients and corresponding cautions or warnings will be required to be carried forward onto other product labels.
The CFIA agrees that additional contact details can be supplied on the label and still achieve the desired outcome of traceability. To reiterate the CFIA's response to similar comments provided in the Consultation Summary – Respondent Comments and CFIA Responses, which was published in response to feedback received from consultation on the CFIA's November 2014 Feed Labelling – Regulatory Framework Proposal, the name and address information on a feed label can be that of the manufacturer, packager, distributor or head office.
Permissible claims
Respondents were generally in agreement with the proposal for establishing a list of permissible claims that could be included on livestock feed labels without requiring mandatory pre-market registration.
CFIA Response:
The CFIA continues to propose that feed labelling, including claims, must be truthful and not misleading.
Following the consultation on the Consolidated Proposal, the CFIA posted a Proposal – Permissible Claims on Feed Labels for public review and feedback in June 2016, with a closing date for comments on July 15, 2016. A separate respondent summary report will be prepared once the feedback the CFIA has received has been analyzed.
Bilingual labelling/International labels
Many respondents expressed concerns regarding the proposed requirement for the bilingual labelling of health and safety-related information including the:
- cost to both create the bilingual label and to have information translated;
- limited access to competent translators;
- accuracy of translations; and
- label may become larger and more complex than necessary.
There was an additional concern expressed regarding unilingual CFIA inspection staff and their capacity to assess and determine compliance for feed labels with information in both official languages.
The CFIA's proposal to allow multilingual or multijurisdictional ("international") labels in the Canadian marketplace also received comments, with respondents generally agreeing with the approach proposed by the CFIA. Concerns were raised regarding the stipulation that international label information must not contradict Canadian labelling requirements, as well as with the timeliness of the approval/registration process for international labels to limit delays.
CFIA Response:
Bilingual labelling of certain consumer products are subject to requirements of the Official Languages Act (OLA). As a follow-up to this consultation and stakeholder feedback, the CFIA has confirmed that the OLA obligations with respect to feed labelling pertain to concerns respecting the health, safety or security of members of the public. However, given that the scope of the Feeds Act extends to the safeguarding of animal health and the environment, the CFIA will proceed with preparing draft regulations that will require the labelling of human and animal health-related safety information (for example, required caution statements on medicated feed labels), in both official languages. Such an approach is considered appropriate at a time when more oversight and prudent use of antimicrobial and other medications is being called for to respond to concerns associated with antimicrobial resistance (AMR).
The CFIA will continue to work with regulated industry to allow for the marketing of products bearing International labels in Canada.
Traceability and Record Keeping
In the Consolidated Proposal, the Codex Alimentarius approach to traceability was proposed to expedite the rapid identification of the origin and movement of feed through the supply chain. Respondents were generally in favour of the proposed traceability approach of tracking feed forward to the immediate customer and backwards to the immediate supplier ("One step forward, one step back"). Concerns were raised by a couple of respondents regarding some challenges with tracking bulk products back to the supplier, and some operations that are not equipped to produce records in electronic format.
CFIA Response:
The CFIA agrees that the Codex Alimentarius approach of "One step forward, one step back" will serve to expedite the rapid identification of the origin and movement of feed through the supply chain.
CFIA further commits to exploring opportunities for training and developing guidance materials for industry and CFIA inspection staff.
Permissions – General comments
General comments on permissions were mostly received from CFIA staff, and indicated a need to consider the internal resources required to put in place new permission schemes for persons or establishments in particular. An additional comment suggested that automating the process would be beneficial.
Permissions – Ingredients
The CFIA received a wide range of comments on a variety of aspects associated with permissions that would apply to feed ingredients.
There was some confusion in stakeholders' comments regarding the proposals to implement a Notice of Submission process (posted once an application is received by the CFIA and before it is evaluated) and a public consultation process on new or modified ingredient approvals (proposed definitions posted for consultation) prior to finalizing the approval.
Based on the comments received, the support for the Notice of Submission process was split. Some respondents felt that this would be beneficial, as stakeholders would know what was going to be entering the market. Others felt that such a process might give away confidential information or prevent companies from applying to have innovative ingredients approved for use in Canada. There were also a couple of questions asking for clarification on what type of information would be posted. There was little support for the consultation on the proposed new or modified ingredient definitions. A couple of questions were raised with respect to protecting confidential information.
The other comments received included the following concerns:
- making the ingredient information public before the ingredient was approved would put the supporting company at a competitive disadvantage;
- competitors would be able to comment on definitions;
- no additional useful information would be provided from this process; and
- it is an unnecessary step and would prolong the approval process.
Only one comment was received in favour of this proposed approach, and it indicated that consultation is an important step to evaluate potential market implications.
There was general support for recognizing foreign approval systems so that ingredients approved in another jurisdiction could be assessed in Canada using a modified pathway. However, there were a number of questions about which countries would be considered equivalent, and what kind of information would be required to support an application.
The section on intended ingredient purpose generated discussion, but no comments were firmly in favour of or opposed to the proposal. Stakeholders asked for clarification on the difference between a purpose and a claim, and suggested that access to alternative products via the low risk veterinary health products pathway provided by Health Canada would be very important.
There was general support for continuing to have a positive list of approved feed ingredients. With respect to foreign approvals, several commenters indicated that the CFIA should adopt the AAFCO's list of ingredients, or consider safety data or approved feed ingredients from other jurisdictions. Several respondents suggested that human food single ingredients (for example, flour; peanut butter) should be allowed for use in feed without going through the feed approval process. One commenter requested that the laboratory assay verification requirement be removed, as it delays ingredient approval without adding any safety benefit. A number of respondents commented on the length of time it currently takes to get a new ingredient approved, and indicated that improved service standards are necessary. Additional comments received indicated that there are some inconsistencies with definitions, or that the definitions listed do not have any clear purpose indicated.
CFIA Response:
The Government of Canada is working with the national and international open government community to create greater transparency and accountability, increase citizen engagement and drive innovation and economic opportunities through open data, open information, and open dialogue (for more information, please refer to Canada's Action Plan on Open Government 2014-16).
The proposed feed ingredient Notice of Submission and pre-approval consultation processes outlined in the Consolidated Proposal are in line with Open Government objectives. The CFIA will develop both processes in consultation with stakeholders as part of the implementation of the modernized regulatory framework.
All feed ingredients will continue to be subject to the approval process to ensure they are safe for use in livestock feeds. However, if an ingredient has already been authorized in a country that the CFIA has recognized as having an authorization process equivalent to that used by Canada; a modified application package may be recognized.
Regarding the positive list of approved feed ingredients, the CFIA is working to update the organization of the list, and correct inconsistencies in terminology and English and French language text. A separate consultation on a proposed revised positive list will follow prior to the publication of a formal regulatory proposal in the Canada Gazette. As a feature of this proposal, the CFIA will be seeking to incorporate the revised positive list (to be referred to as the "Canadian Feed Ingredient Table") in keeping with the CFIA's Incorporation by Reference Policy. While respondents have not generally supported the Agency's proposal to consult on feed ingredient descriptions prior to their final approval, the Incorporation by Reference Policy does direct that an appropriate public consultation be provided as part of the change management process for documents to be referenced in regulatory frameworks.
Permissions – Mixed feeds
In the Consolidated Proposal, the CFIA proposed that the number of mixed feed that requires pre-market registration would be reduced; however, some types of feeds, namely milk replacers, feeds delivered via water, medicated minerals and flavouring agents would continue to require registration. One respondent indicated that registration should be limited to ingredients, and that neither imported nor domestic mixed feeds should require registration.
A number of respondents commented that milk replacers should not require registration, and gave the following reasons in support of this suggestion:
- other feeds for young animals are currently exempted from registration;
- the same nutrient standards would apply to milk replacers; and,
- this approach would be consistent internationally.
A few questions were asked seeking clarification of whether specific types of feeds would require registration under the proposal. In addition, one respondent indicated that they would like to see voluntary registration to allow companies to protect their proprietary products.
The Consolidated Proposal also proposed that Table 4 be removed from the Regulations and replaced with maximum values for nutrients that pose a risk of harm. In addition, with the removal of Table 4, it was proposed that customer formula feeds and consultant formula feeds would no longer be required.
As previously mentioned, there was general support for removal of Table 4 from the modernized Regulations, with additional comments indicating that any maximum values should be based on health and safety, not nutritional adequacy.
There was general support for the removal of consultant and customer formula feed categories from the Regulations. Respondents felt there would not be a need for these types of feeds (acting as exemption from registration) if the nutrient maximum limits are appropriate. A number of questions of clarification were asked, especially with respect to on-farm feed manufacture and manufacture of medicated feeds. In addition, with respect to the proposal for Veterinary Prescription feeds, there were a number of questions regarding whether prescriptions would need to be bilingual and the role of the veterinarian with respect to nutrient content.
CFIA Response:
Based on the feedback provided, the CFIA re-examined its policy intent with respect to requiring registration for milk replacers, and has concluded that they will be exempted from mandatory registration like other complete feeds.
The CFIA will have further consultation on proposed values for nutrient maximums for feeds.
Permissions – Persons or establishments
As illustrated in Table 3 below, the Agency received a number of written comments relating to Permissions – Persons or establishments. The CFIA had not elaborated any measures in this regard in any of its previous consultation proposals. Respondents' comments varied, including 1, 2 or all 3 of the subjects outlined in this section.
| Permissions – Subject | # of respondents |
|---|---|
| Persons or establishments (domestic) | 23 |
| Persons or establishments (importers) | 17 |
| Persons or establishments (exporters) | 15 |
Although stakeholders were prompted in the Consolidated Proposal to comment specifically on proposals regarding requirements for permissions in the context of domestic, import and export trade, the CFIA received questions from several respondents regarding more administrative aspects of the proposed permissions regime, such as:
- what will be the fee for a permission?;
- what will be the duration of a permission?;
- what will be the application requirements, and will they impose additional requirements on regulated parties (for example, additional documentation)?;
- what criteria will the CFIA use to issue permissions?;
- will preventive control plans (PCPs) have to be submitted and approved by the CFIA?;
- what frequency of transactions will trigger a requirement for a regulated party to have a preventive control plan as a condition for being able to obtain a permission?; and
- how will decisions be made in the event enforcement actions are to be taken by the CFIA on permissions (suspensions, cancellations)?
CFIA Response:
Information concerning some administrative aspects of the CFIA's permissions system has been provided in Annex A: Permissions processes, of the integrated Agency Inspection Model, such as applying for/obtaining a permission, the process for issuing a permission, the process for suspending a permission, etc.
For other aspects that are not covered in the model, the CFIA is preparing a Permissions and Registrations policy as well as modernized User Fees and Service Standards. The CFIA expects to consult with stakeholders on both of these initiatives during 2016-17.
Permissions – Persons or establishments (domestic)
Much like what the CFIA heard during the town hall public meetings staged across Canada during February-March 2016, many respondents indicated that the CFIA should not limit the permission requirements for persons or establishments solely doing business across provincial boundaries. Reasons cited by respondents for this position included:
- the proposed permission requirements go against some of the stated objectives of the regulatory modernization initiative (e.g., reducing regulatory burden, increasing the focus on health and safety); and,
- the proposed permission requirements on some (but not all) regulated parties would create inequities between regulated parties regarding:
- additional costs for some regulated parties related to fees for permissions; and
- differing inspection strategies and enforcement approaches applied by the CFIA for some regulated parties.
CFIA Response:
The CFIA will prepare draft regulations with respect to permission requirements for persons or establishments involved in the inter-provincial trade of feeds and feed ingredients in keeping with the principles of the integrated Agency Inspection Model, the proposed Safe Food for Canadians Regulations and within the scope of authorities provided to the CFIA in the Feeds Act. The CFIA will also work with legal counsel to explore mechanisms to provide, if possible, permissions to regulated parties who do not otherwise require a permission, but may voluntarily wish to have one.
Permissions – Persons or establishments (imports)
In the Consolidated Proposal, the CFIA presented stakeholders with three options to consider regarding the oversight of feed importers or imported products in a modernized framework:
- Option 1: Continue with some manner of product permissions (ingredient and mixed feed registration) for import control (modified status quo);
- Option 2: Require importers in Canada to obtain a permission from the CFIA, with a preventive control plan (PCP) as a condition; and
- Option 3: Authorize operators or establishments in other countries (by way of permissions) to export feeds directly to Canadian customers.
From the responses received, no clear consensus emerged as to which option or combination of options would be favoured by the broad range of stakeholders who commented on the proposal:
- five respondents indicated that option 1 would still provide an effective oversight approach to feed product importers, but one respondent commented that, "Option 1 does not provide for a level playing field and also requires substantial CFIA resources";
- three respondents supported option 2, and three respondents supported a combination of options 2 and 3; and
- two respondents did not support option 3, commenting that "[this option] puts an unfair burden on the Canadian manufacturer and not the company importing a foreign registered [product]", and "with limited resources available to the CFIA to conduct the required inspections, option 3 should not be considered for import".
Three respondents also suggested that the CFIA should issue permissions to laboratories in Canada to better enable the importation of feed samples for analysis only. The current Regulations do not have specific exemptions or conditions for imports of feeds for this purpose
CFIA Response:
The CFIA will prepare draft regulations with respect to permission requirements for importers and imported products to provide as much flexibility as possible under the Feeds Act to accommodate different oversight options, particularly if it will take some time to enable the delivery of CFIA permissions to foreign exporters as proposed in option 3.
Permissions – Persons or establishments (exports)
In the Consolidated Proposal, the CFIA put forward a more structured approach to the oversight of feed exporters and exported products in a modernized framework, where:
- exporters of feed would have to obtain a permission from the CFIA;
- feeds for export would have to be manufactured in accordance with a preventive control plan (PCP) developed, implemented and maintained by the exporter;
- feeds for export would be required to meet the minimum domestic safety standards as set out in the Regulations;
- the CFIA would provide exemptions to non-health and safety standards to provide exporters with flexibility to meet trading partners' requirements that may differ from Canadian domestic requirements; and
- the CFIA would provide export certificates where trading partners require certification and the products destined for export markets meet the importing countries' requirements.
As with responses regarding the CFIA's proposed options for the oversight of importers/imported products, no clear consensus emerged with respect to the approach proposed by the CFIA for exporters and feed products destined for export markets. Comments from respondents included the following:
- five respondents did not support the CFIA's proposal to require exporters to obtain a permission to do so because, for example, it "seems redundant to require exporters to obtain a permission if there is a PCP in place";
- three respondents did support the proposal that all feeds manufactured for export be subject to a PCP, while one did not;
- three respondents did not support the proposal that feeds for export should meet minimum domestic safety standards as established in the Regulations, while one did; and
- three respondents indicated it would be important to exporters that the CFIA issue export certificates.
CFIA Response:
In keeping with the principles of the integrated Agency Inspection Model (iAIM), the CFIA will prepare draft regulations with respect to permission requirements for exporters and feeds for export trade to provide as much flexibility as possible on exempting such feeds from the non-health and safety requirements of the Regulations.
Next steps
The CFIA will prepare a formal regulatory proposal for publication in the Canada Gazette Part I in 2017, which will incorporate the comments received on all the consultation proposals, public meetings, stakeholder workshops and submissions, and other outreach activities that have been used over the course of the project.
In the interim, the following proposed documents to be incorporated by reference in the modernized regulatory framework have or will be prepared and shared with stakeholders for review and comment:
| # | Document name |
|---|---|
| 1 | |
| 2 | Permissible Claims on Feed Labels |
| 3 | Nutrient Guarantees on Feed Labels |
| 4 | Veterinary Biologics in Feeds |
| 5 | Oversight of Weed Seeds in Feeds |
| 6 | Maximum Nutrient Levels in Feeds |
| 7 | Maximum Contaminant Levels in Feeds |
| 8 | Positive List of Approved Ingredients |
| 9 | Compendium of Medicating Ingredient Brochures (CMIB) |
The proposed documents have or will be distributed and posted on the Livestock Feed Consultations on Proposed Regulatory and Policy Changes webpage for stakeholder review and comment. Respondent feedback summary reports have or will be prepared, distributed posted on the same webpage with their corresponding proposals with the outcomes of these additional consultations. The finalized documents will be included as part of the modernized regulations package for pre-publication in the Canada Gazette.