Quality Management System Requirements For Facilities Receiving and Handling Regulated Non-Propagative Potatoes and Related Potato Articles, Including Associated Soil
Table of contents
- Contact and Review
- Endorsement
- Distribution
- Introduction
- 1.0 Scope
- 2.0 References
- 3.0 Definitions, abbreviations and acronyms
- 4.0 CA approval process
- 5.0 Responsibilities of the approved facility
- 6.0 Quality Management System Manual
- 7.0 Training of employees
-
8.0 CFIA evaluations and audits
- 8.1 Evaluation
- 8.2 Surveillance audit
-
8.3 Non-conformances
- 8.3.1 Critical non-conformance
- 8.3.2 Major non-conformance
- 8.3.3 Minor non-conformance
- 8.3.4 Observations
- 8.3.5 Suspension of a CA
- 9.0 Enforcement measures
-
10.0 Internal audits
- 10.1 Frequency
- 10.2 Documenting the internal audit
- 10.3 Corrective actions
- 11.0 Record keeping and document verification
- Appendix 1 Application for Approval under a Compliance Agreement (CA) with the Canadian Food Inspection Agency (CFIA)
- Appendix 2 CFIA Evaluation Checklist
- Appendix 3 CFIA Surveillance Audit Checklist
- Appendix 4 Report (D-96-05 & QSM-09)
- Appendix 5 CFIA Corrective Action Request (CAR)
- Appendix 6 List of Required Records and Documents
- Appendix 7 Required elements of the Quality Management System Manual of an Approved Facility
- Appendix 8 Guidelines for the Transport and Disposal of Regulated Articles and Cleaning Requirements
Contact and review
This document will be updated as required. For further information or clarification, please contact the Canadian Food Inspection Agency (CFIA).
Endorsement
Approved by:
![]()
Chief Plant Health Officer
Distribution
- Plant Health Directive e-mail notification
- Other government organizations (Federal, Provincial, Municipal) (determined by Author)
- National industry organizations and stakeholders (determined by Author)
- Internet
Introduction
General requirements for the importation and domestic movements of non-seed potatoes are prescribed in CFIA's Directive D-96-05, Phytosanitary requirements for the importation and domestic movement of non-propagative potatoes (Solanum tuberosum) and related potato articles, including associated soil. D-96-05 introduces the concept of a Compliance Agreement (CA) as one of the options available to permit the movement of regulated potato articles in Canada.
A facility must be approved by the CFIA under a CA in order to be issued a Permit to Import or a Movement Certificate for the purpose of receiving and/or handling regulated articles as described in D-96-05.
1.0 Scope
The purpose of QSM-09 is to provide detailed information on the CA option as described in D-96-05. QSM-09 outlines the requirements for a facility to apply for approval under a CA, and for the CFIA to audit the facility. In particular, QSM-09 provides instructions and a template for the development of a Quality Management System Manual (herein referred to as the Manual).
QSM-09 is intended for use by both CFIA inspection staff and facilities seeking approval or already approved under a CA.
2.0 References
- Canadian Food Inspection Agency Fees Notice, Canada Gazette, Part I
- D-96-05: Phytosanitary requirements for the importation and domestic movement of non-propagative potatoes (Solanum tuberosum) and related potato articles, including associated soil.
- Plant Protection Act (S.C. 1990, c.22)
- Plant Protection Regulations (SOR/95-212)
- PI-016 - Procedure for inspecting regulated articles for freedom from soil, plants, plant parts and related matter
3.0 Definitions, abbreviations and acronyms
Definitions for terms used in the present document can be found in the Plant Health Glossary of Terms.
4.0 CA approval process
4.1 Application for CA approval
A facility seeking approval under a CA must be willing to adhere to the administrative and operational requirements described in QSM-09 and D-96-05. A signed Application for Approval under a CA with the Canadian Food Inspection Agency (Appendix 1) and a copy of the Manual must be sent to the local CFIA office for review. A list of local CFIA offices can be found on the CFIA website.
4.2 Official signature
The person responsible for the facility's quality management system must sign the Application for Approval under a CA with the Canadian Food Inspection Agency (Appendix 1).
4.3 Evaluation of applications
The CFIA will review and compare the facility Manual to the CA requirements as specified in D-96-05 and QSM-09. An evaluation of the facility will be conducted by the CFIA.
4.4 Approval process
Provided the Manual meets the CA requirements and the evaluation of the facility is satisfactory, the CFIA will sign the application form (Appendix 1) and the facility will be considered approved. A copy of the signed application must be placed in an appendix at the end of the Manual.
4.5 National list of CA-approved facilities
Listing of the facility in the national list of CA-approved facilities is mandatory. The list will be kept internally and will not be disclosed to individuals other than CFIA employees.
4.6 CA approval's lifespan
For facilities handling regulated articles year-round, the CA continues to be valid if a surveillance audit has been completed within the last 90 days, and all corrective actions (if any) have been addressed to the satisfaction of the CFIA.
The CA is no longer valid and the facility must complete an Application for Approval under a Compliance Agreement (CA) with the Canadian Food Inspection Agency (CFIA) (Appendix 1) if:
- An audit has not been completed in the last 90 days;
- The facility has been suspended;
- The facility has voluntary withdrawn from the program;
- The Import Permit or the Movement Certificate has been cancelled.
Note: The local CFIA office must be notified upon arrival of the first load of regulated articles. A surveillance audit is required prior to any movement of regulated articles to the facility. A review of the Manual may also be required.
4.7 Re-applying for CA approval
A facility that has voluntarily withdrawn from a CA may re-apply for CA approval following the instructions outlined in section 4.1.
A facility for which the CA has been suspended by the CFIA (per section 8.3.5) may re-apply for CA approval, provided all requested corrective actions have been implemented to the satisfaction of the CFIA. An updated version of the Manual and a detailed report on corrective actions taken by the facility must accompany the application form (Appendix 1). Additional CFIA scrutiny including an increased audit frequency will also be implemented following the approval. Once on-going conformance has been demonstrated to the satisfaction of the CFIA, the facility will be assigned the regular audit frequency.
5.0 Responsibilities of the approved facility
The approved facility must comply with all the provisions of D-96-05 and QSM-09, including:
- Planning, drafting and maintaining the Manual
- Implementing the provisions of the Manual.
- Circulating all updated versions of the Manual to the CFIA and the facility's staff.
- Obtaining the CFIA's approval before implementing any changes to the Manual.
- Conducting internal audits and making results available to the CFIA.
- Cooperating with the CFIA's auditors.
- Applying corrective actions as discussed and mutually agreed with the CFIA, and consequently updating the Manual to reflect corrections to administrative or operational procedures.
- Employing competent staff in sufficient numbers to carry out the requirements of the Manual.
- Training all staff members involved in receiving or handling the regulated articles in accordance with the provisions of the Manual. All staff involved must be aware of the phytosanitary requirements associated with the facility's CA approval.
- Identifying a person responsible for the facility's quality management system.
- Develop a contingency plan in case of an accidental spillage or overflow of the regulated articles.
- Ensure all third parties contractors comply with the requirements of D-96-05 and QSM-09.
- Informing the CFIA of receiving schedules for regulated articles.
6.0 Quality Management System Manual
The Manual should be designed so that full regulatory compliance is met through fulfillment of the provisions of the Manual. It is the facility's responsibility to develop its own Manual. The Manual will be used as a basis for evaluation and surveillance audits conducted by the CFIA, as well as internal audits conducted by the approved facility. A Manual must be kept up-to-date at all times, reflecting the facility's organizational and operational plans and the associated activities. Modifications to the Manual must be documented according to a protocol determined by the approved facility and described in the Manual. Approved facilities must seek CFIA approval prior to implementing any changes made to the Manual. Timelines for updating the Manual after findings of non-conformance are the same as the timelines for responding to a specific non-conformance (i.e., next audit for a minor, 2 weeks for a major etc.)
Appendix 7 outlines all expected elements to be found in the Manual of an approved facility. Note that some sections listed in Appendix 7 may not apply for some facilities.
In addition, Appendix 8 provides general guidelines for the movement and disposal of regulated articles and cleaning of facilities equipment. These elements should also be taken under consideration into the Manual.
7.0 Training of employees
Timely and effective training of all individuals involved in implementing the requirements of the Manual is essential. The approved facility must develop a training strategy and training plans must be documented and implemented. This includes relaying information contained in the Manual, D-96-05 and QSM-09, as well as any information pertinent to changes made to the Manual, D-96-05 and QSM-09. All CA-related training delivered and received must be recorded. Training records must indicate training dates, names of trainers and trainees, training type and content, and whether the training was completed satisfactorily. Training records must also include any additional training needs identified.
8.0 CFIA evaluations and audits
CFIA evaluations and audits are verifications that the facility conforms to the requirements of both the Manual and the D-96-05. CFIA audits may be scheduled and carried out during or after the period in which the facility is receiving potato articles from regulated areas.
CFIA inspection staff will use checklists to conduct evaluations and audits (Appendices 2 and 3). The person responsible for the facility's quality management system must be present and available to help during all scheduled CFIA activities at the facility. This includes allowing the CFIA inspectors to examine records and documents, collect samples, inspect articles and equipment, observe processes and interview facility staff.
Evaluation and audit findings will be compiled by the CFIA in a report (Appendix 4). Non-conformances will be identified and recorded in the evaluation and audit checklists (Appendices 2 and 3), and a Corrective Action Request (CAR) will be issued to the facility (Appendix 5) for any identified non-conformances. The facility is responsible for the implementation and documentation of corrective actions to address non-conformances within the time frame specified in the CAR. Corrective actions will be verified by the CFIA.
8.1 Evaluation
The CFIA evaluation is conducted prior to any movement of regulated articles into the facility and is a scheduled systematic examination of a new CA applicant's facility, which verifies if the facility is capable of meeting program requirements. It includes a review of the Manual and its proposed implementation plan (Appendix 2) and must be completed prior to CA approval. The evaluation will be scheduled after the facility has applied for CA approval (Appendix 1) and submitted a copy of their Manual to the CFIA. All non-conformances found during the course of the evaluation must be addressed before the facility is approved. Failure to address non-conformances and update the Manual accordingly will result in rejection of the application for CA approval.
8.2 Surveillance audit
The CFIA surveillance audits are reviews of the organizational structure, procedures, processes and resources used by the approved facility to fulfill all CA requirements, including the verification that the Manual and the corrective actions are implemented as planned (Appendix 3). Surveillance audits also include the review of all internal audit reports and associated records produced by the approved facility.
In general terms, the CFIA will conduct at least one surveillance audit during the period when the facility is receiving and handling regulated articles, with a minimum of one surveillance audit every three months. Ideally, the first surveillance audit will be performed within a week of receiving the first load of regulated articles. The last surveillance audit will be conducted immediately after the period during which regulated articles are handled by the approved facility. The purpose of the last surveillance audit is to verify proper cleanup practices in accordance with the Manual. For facilities handling regulated articles year-round, a specific schedule for clean-up of all equipment and areas used for receiving, conveying, storing and processing the regulated articles must be kept on record and will be used as a basis for at least one targeted CFIA surveillance audit per year.
Unsatisfactory findings during the surveillance audit will be dealt with in accordance with the non-conformance scheme as prescribed in section 8.3.
8.3 Non-conformances
During the course of a CFIA audit or evaluation, any procedures, documentation, or articles that are found to be in contravention of the standards of the quality management system implemented by the facility are considered to be a non-conformance; this includes any deficiencies of the procedures in meeting the requirements of D-96-05 and QSM-09 or violations of the provisions of D-96-05, QSM-09 or the Manual.
Non-conformances can be classified into three types: critical, major, or minor. The classification of non-conformances is based on the evaluation of the associated phytosanitary risk and the resulting threat to the integrity of the quality management system of the approved facility. In the event of a dispute over the classification of a non-conformance, the CFIA's decision will be final.
For every non-conformance found during the course of a CFIA audit or evaluation, a Corrective Action Request (CAR) will be issued to the approved facility (Appendix 5), and the implementation of corrective actions will be expected and monitored by the CFIA. The approved facility must seek approval from the CFIA prior to implementing remedial actions to address CARs.
The identification of non-conformances and/or the unsatisfactory management of the associated CARs may increase the CFIA audit frequency for a period to be determined by the CFIA, and may result in suspension of a facility's CA.
8.3.1 Critical non-conformance
A critical non-conformance is any single audit finding that reveals that the integrity of the quality management system of the approved facility is in jeopardy. The facility will be immediately suspended from the program if any critical non-conformances are found by the CFIA. Suspended facilities can re-apply for CA approval as per section 4.7.
Critical non-conformances include, but are not limited to:
- Facility operating without implementing the requirements of D-96-05 and QSM-09 for the movement of potatoes originating from a regulated area.
- Employees of the facility are not aware of the requirements of D-96-05.
- Failure to take corrective action on a major non-conformance during the specified timeframe.
- Failure to perform any internal audits.
- Records for the program are unavailable or do not exist.
8.3.2 Major non-conformance
A major non-conformance is any isolated incident of non-conformance which does not immediately impact on the integrity of the quality management system of an approved facility. Corrective actions must be completed in a CFIA-approved manner within the time frame specified by the CFIA, which shall not exceed a maximum of two weeks. If two or more major non-conformances are detected during a CFIA audit, or if the facility fails to carry out the required corrective actions within two weeks, the non-conformance will be assessed as critical and the facility's CA will be immediately suspended. Suspended facilities can re-apply for CA approval as per section 4.7.
Major non-conformances include, but are not limited to:
- Facility operating with significant changes to their procedures that have not been approved by CFIA.
- Employees of the facility who are involved with implementing the quality procedures are not sufficiently trained.
- Loads of regulated potato articles received by the facility are not recorded in a log.
8.3.3 Minor non-conformance
Minor non-conformances are incidents that do not immediately and/or significantly affect the integrity of the quality management system of an approved facility, but that could lead to a major non-conformance if left unaddressed. Minor non-conformances must be addressed in a CFIA-approved manner before the next surveillance audit, or within the time frame specified by the CFIA. Should the facility fail to complete the corrective actions in the specified time frame, the non-conformance could lead to a major non-conformance.
If three or more minor non-conformances are detected in any one CFIA audit, this is considered equivalent to one major non-conformance. Therefore, four minor non-conformances are equal to one major plus one minor non-conformance. Similarly, six minor non-conformances are equal to two major non-conformances, which constitute a critical non-conformance; the facility's CA would therefore be immediately suspended.
Minor non-conformances include, but are not limited to:
- Facility operating with minor adjustments to their procedures, incorporated or not in their quality manual, which have not been reviewed and accepted by CFIA.
- Facility record-keeping is inadequate but records essential to the integrity of the phytosanitary standard (e.g. receiving log records) are complete.
- One load of regulated potato articles received by the facility was not recorded in the log.
8.3.4 Observations
The CFIA's observations are points or practices which may be noted during audits and could be used to improve the Manual. An observation may be used to identify a situation of concern that does not warrant a CAR, or to highlight, suggest or reinforce particular practices.
Observations may include but are not limited to:
- Suggest computer backup of files be done on a more regular basis.
- Excellent record keeping.
- Internal audit reports are being completed and are available, but an updated copy of the audit report template should be added to the Manual.
8.3.5 Suspension of a CA
The suspension of a CA must occur in consultation with the CFIA Regional Program Officer (RPO) and the CFIA Area Program Specialist.
The CFIA will suspend a CA if:
- One critical non-conformance is identified by the CFIA; or
- The equivalent of one critical non-conformance is identified (i.e. two major, six minor, or one major plus three minor); or
- The approved facility fails to address a major non-conformance; or
- The approved facility voluntarily withdraws from a CA.
Immediately following the suspension of a facility's CA, the facility must terminate all importation/receiving and handling of regulated articles. The CFIA will suspend all Permits to Import and Movement Certificates associated with the regulated articles to prevent their movement to and from the suspended facility. Additional regulatory controls may be imposed on the suspended facility by the CFIA in order to alleviate any other phytosanitary risks associated with the non-conformance or the situation.
The facility's name and contact information will be removed from the National List of CA-Approved Facilities.
A suspended facility can re-apply for CA approval following instructions in section 4.7.
9.0 Enforcement measures
In addition to the fulfillment of the provisions of D-96-05 and QSM-09, facilities approved under a CA must ensure they comply with the Plant Protection Act, the Plant Protection Regulations and any additional requirements that apply to the regulated area from which the potato article is originating. The CFIA may take enforcement measures including prosecution for any violations of the above requirements.
10.0 Internal audits
10.1 Frequency
The person responsible for the approved facility's quality management system must conduct internal audits, or designate and supervise employees to perform internal audits. In general terms, it is expected that internal audits be conducted at least twice during the period during which the approved facility is receiving and handling regulated articles, with a minimum of one internal audit every month. Internal auditors shall not audit their own work. Examples of audit checklists can be found in Appendices 2 and 3.
Internal audits do not need to be performed during the period when an approved facility does not receive or handle regulated articles subject to the CA, unless otherwise specified in the Manual
Internal audits include, but are not limited to:
- The assessment of the adequacy and effectiveness of the facility's processes as outlined in their Manual in meeting the CA requirements prescribed in D-96-05 and QSM-09.
- The verification of whether the required CA-related documentation is sufficient, current and readily available to staff.
- The verification whether the facility's quality management system is operating in accordance with the specified requirements, including the performance of all staff identified in the Manual.
- The verification that effective corrective action plans have been developed and properly implemented for all non-conformances identified.
- The evaluation of the competency of employees in carrying out duties and responsibilities as outlined in the Manual.
- The verification that the facility's record-keeping activities comply with the provisions of the Manual and are sufficient to ensure proper traceability and segregation of the regulated articles.
- The verification that actions are taken on all outstanding non-conformances or CFIA CARs.
10.2 Documenting the internal audit
Records of every internal audit must be kept for a period of ten years. An internal audit report must be prepared within three working days of performing the internal audit, detailing any non-conformances, remedial action plans, corrective actions implemented and opportunities for improvement that related to CA requirements. Internal audit reports must be made available to the local CFIA office responsible for the surveillance audits. Internal audit reports and associated records will be review by the CFIA during surveillance audits.
10.3 Corrective actions
Activities or articles that are found to be in violation of the provisions of D-96-05, QSM-09 or the Manual are considered non-conforming. Non-conformances detected during internal audits must be documented in internal audit reports. Corrective actions must be implemented for each non-conformance detected by the approved facility. Remedial action plans and the implementation of corrective actions must also be documented in internal audit reports.
Non-conformances found during internal audits must be identified as being "critical", "major" or "minor", in accordance with section 8.3. The implementation of remedial action plans and the associated corrective actions must be prioritized based on the type of the non-conformance. Internal audit reports must include detailed instructions on how to prevent recurrences of the non-conformances, generally requiring that the Manual be amended and staff be informed accordingly. It is the responsibility of the facility to inform CFIA of any critical non-conformances found during an internal audit no later than the next business day following the finding.
11.0 Record keeping and document verification
Record keeping is a key CA requirement. The approved facility must keep records of all organizational and operational processes and procedures used to fulfill all CA requirements. Internal and CFIA auditors will review these records to verify if the facility's quality management system as described in its Manual, including the corrective actions, is implemented as planned. As well, the verification of an approved facility's records will help the CFIA determine if a facility satisfies all requirements stated in D-96-05 and QSM-09. Records must be kept on the premises of the approved facility and must be available to the CFIA upon request at any time. CA-required records must be kept for a minimum of ten years. CA-required records relate to receiving, rejecting, storing, handling, processing and shipping regulated articles, disposing of by-products, cleaning activities, training of staff, identifying and remedying non-conformances, internal and CFIA audit reports, tracking amendments to the Manual, etc. Consult Appendix 6 for list of all CA-required records and documents.
In addition, up-to-date copies of the following documents must be readily accessible for use by all facility staff and contractors involved in the management, processing or handling of regulated articles:
- Plant Protection Act and Plant Protection Regulations.
- D-96-05: Phytosanitary requirements for the importation and domestic movement of non-propagative potatoes (Solanum tuberosum) and related potato articles, including associated soil.
- QSM-09: Compliance agreement - Quality system requirements for facilities receiving and handling regulated non propagative potatoes, related potato articles and the associated soil.
- The Manual
- Any other regulatory documents that relates to the areas from which the imported or received potato article is originating.
Appendix 1: Application for Approval under a Compliance Agreement (CA) with the Canadian Food Inspection Agency (CFIA)
As described in D-96-05 and QSM-09 Under the authority of the Plant Protection Act and Regulations of Canada
Please check one:
![]() New applicant
New applicant
![]() Renewal (good compliance status)
Renewal (good compliance status)
![]() Re-application (following voluntarily withdrawal from the CA)
Re-application (following voluntarily withdrawal from the CA)
![]() Re-application (following CA suspension by the CFIA)
Re-application (following CA suspension by the CFIA)
Company name
Facility address
Contact Person
Title or position
Phone
Fax
Cell
Applicant's statement:
I agree to act as the person responsible for the above facility's quality management system.
I have read and agree to comply with all provisions of D-96-05 and QSM-09. In particular, I agree:
- To implement the provisions of the Manual and keep it up to date.
- To circulate all updated versions of the Manual to the CFIA and the facility's staff.
- To seek the CFIA's approval before implementing any changes to the Manual.
- To conduct internal audits and make the results available to the CFIA.
- To cooperate with the CFIA's auditors.
- To apply corrective actions as discussed and mutually agreed to with the CFIA, and update the Manual accordingly.
- To employ competent staff in sufficient numbers to carry out the requirements of the Manual.
- To train all individuals involved in implementing the provisions of the Manual.
- To identify a person responsible for the facility's quality management system.
- To develop a contingency plan in case of an accidental spillage or overflow of the regulated articles.
- To ensure all third parties contractors comply with the requirements of D-96-05 and QSM-09.
- To inform the CFIA of receiving schedules for regulated articles.
I will contact the local CFIA office as soon as a date for receiving the first load of regulated articles has been confirmed.
I have read and understand all conditions in the above statement.
![]()
Signature
![]()
Date
![]()
Printed Name and Title
Approval Statement from the CFIA:
In my capacity as CFIA Inspector or Regional Program Officer authorized under the Plant Protection Act, and further to my review of the applicant's Manual and results of the evaluation audit of the facility, I authorize the above facility to be approved under a CA with the CFIA in order to implement the provisions of their Manual for the following period:
Period importing (active): from ![]() to
to ![]() .
.
![]()
Signature & printed name
CFIA Inspector or Regional Program Officer
![]()
Date
![]()
Signature & printed name
CFIA Area Program Specialist
![]()
Date
This application form must be submitted to the local CFIA office. A list of local CFIA offices can be found at the CFIA website.
Appendix 2: CFIA Evaluation Checklist (D-96-05 & QSM-09)
The following checklist should be used during the evaluation of the facility.
Company name
Facility address
Contact Person
Title or position
Phone
Cell
Fax
CFIA Evaluators
Date of Evaluation
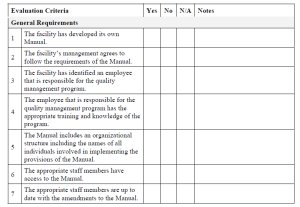
Evaluation Checklist (D-96-05 & QSM-09)
This checklist is designed to be completed by a CFIA inspector. The Evaluation Checklist is split into 2 sections. The first section is for recording the general details about the CFIA Evaluation, e.g. the company name and address, evaluation date, etc. The second part of the checklist is for recording the details of the facility's conformance with the evaluation criteria. The sections listed in the checklist are the following:
- General Requirements
- Training
- Internal Audit
- Receiving and Handling of Regulated Article
- Handling and Disposal Rejected Loads
- Storing, Tracking and Labelling
- Grading
- Washing and Brushing
- Peeling and/or Cooking
- Packing and Repacking
- Sprout Inhibition
- Collection within the Facility
- Final Disposition of By-Products
- General
Appendix 3: CFIA Surveillance Audit Checklist (D-96-05 & QSM-09)
The following checklist should be used during the surveillance audit of the facility.
Company name
Facility address
Contact Person
Title or position
Phone
Cell
Fax
CFIA Auditors
Date of Audit
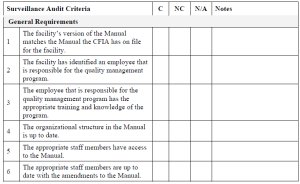
CFIA Surveillance Audit Checklist (D-96-05 & QSM-09)
This checklist is designed to be completed by a CFIA inspector. The Surveillance Audit Checklist is split into 2 sections. The first section is for recording the general details about the CFIA Surveillance Audit, e.g. company name and address, audit date, etc. The second part of the checklist is for recording the details of the facility's conformance with the audit criteria. The sections listed in the checklist are the following:
- General Requirements
- Training
- Internal Audit
- Receiving and Handling of Regulated Article
- Handling and Disposal Rejected Loads
- Storing, Tracking and Labelling
- Grading
- Washing and Brushing
- Peeling and/or Cooking
- Packing and Repacking
- Sprout Inhibition
- Collection within the Facility
- Final Disposition of By-Products
- General
Appendix 4: Report (D-96-05 & QSM-09)
Importation and Domestic Movement of Non-Propagative Potatoes
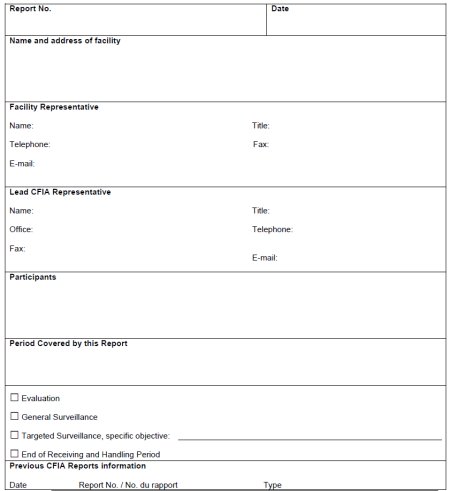
Report (D-96-05 & QSM-09)
This report is designed to be completed by a CFIA inspector. The Report summarizes the results of the CFIA Evaluation or the CFIA Surveillance Audit. The report also lists:
- Report no.,
- Name and address of facility,
- Facility representative information,
- Lead CFIA representative information,
- Participants,
- Period covered by the report,
And all the Corrective Action Requests issued to the company as a result of the CFIA Evaluation or the CFIA Surveillance Audit.
Appendix 5: CFIA Corrective Action Request (CAR) (D-96-05 & QSM-09)
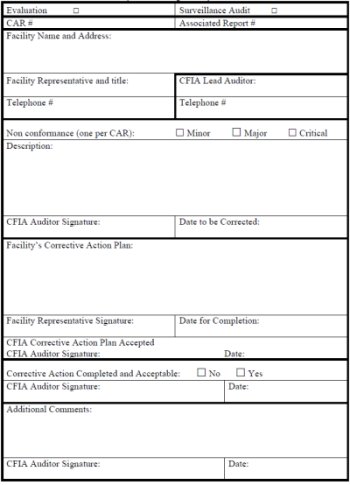
Corrective Action Request (D-96-05 & QSM-09)
The Corrective Action Request is designed to be issued by a CFIA inspector. The Corrective Action Request is split up into 5 parts. The first part is for recording the general details about the CFIA Evaluation or CFIA Surveillance Audit. The second part describes the details of a non-conformance found during a CFIA Evaluation or a CFIA Surveillance Audit. The third part is for the facility's corrective action plan. This section has to be sign by the facility representative. The fourth part is for the CFIA inspector to sign the Corrective Action Request once the corrective action plan has been completed. The fifth and final part of the Corrective Action Request is for additional comments and the signature box.
Appendix 6: List of Required Records and Documents
The following records, logs and documents must be kept for a period of ten years.
Records to be kept up to date:
- Staff and contractors responsibility list;
- Training records;
- Shipment reception log;
- Shipment rejection log;
- Logs and records associated with storing, handling, grading, processing, packing, shipping, sprout inhibiting and other activities;
- Logs and records for the collection and disposal of regulated articles and by-products;
- Cleaning records;
- Internal audit records;
- Records of internal and CFIA audit non-conformances and corrective actions;
- Manual updates.
Documents to be available at any time (electronic copies are acceptable):
- Movement Certificates;
- Corrective Action Requests;
- Permits to Import;
- Bills, shipping slips, treatment certificates or any other document that relates to tasks required by the Manual;
- Previous versions of the Manual;
- Internal audit reports;
- Any documents sent by the CFIA (letters, reports, etc.)
Appendix 7: Required elements of the Quality Management System Manual of an Approved Facility
The following information must be included in the Manual of an approved facility under a CA in accordance with the provisions of D-96-05 and QSM-09. Some requirements may not apply.
A. Format of the Manual
The front page of the Manual must display the following information:
- The facility's legal and business name.
- The complete address (es) of the premises where the facility will be receiving and handling the regulated articles subject to the CA.
- The suggested document title "[insert company name] Quality Management System Manual for the implementation of the CFIA CA requirements in order to receive and handle regulated potato articles as described in D-96-05 and QSM-09".
- The date and/or version number of the document.
The following information should be recorded on each page of the Manual:
- On the lower left-hand side: version date and/or number.
- On the lower right-hand side: "page X of Y" pagination.
B. Generalities
B1. Management Endorsement
Facility management must sign and date a statement in the Manual, indicating that they agree to operate in accordance with the terms of the Manual, D-96-05 and QSM-09. Facility's management must identify one employee responsible for the facility's quality management system as a main contact for the CFIA, including at least one alternate person. Names of representatives must be clearly spelled out.
B2. Facility Description and Administrative Structure
The Manual must briefly describe the facility's main business lines. All activities associated with CA requirements prescribed in D-96-05 and QSM-09 must be described in detail. The Manual must list all employees involved in these activities, including a description of their respective responsibilities. The name of all individuals involved in implementing the provisions of the Manual must appear in the Manual; this includes all hired third-party contractors. An organizational chart must be included in an appendix to the Manual.
B3. Training of Employees
All training plans pertinent to the implementation of the Manual requirements must be described in the Manual. This includes a description of the process by which the facility deals with and communicates changes to the Manual. Training records must be completed and kept. A template for training records must be included in an appendix to the Manual.
B4. Internal Audits
The facility's plan for conducting internal audits must be described in the Manual. This includes a description of the audit frequency and the associated operational procedures. Internal audit reports must be completed and kept. A template for internal audit reports must be included in an appendix to the Manual.
B5. Updating the Manual
The Manual must describe how to update the Manual. All modifications to the Manual should be recorded in a tracking sheet to be included in an appendix to the Manual.
B6. Record Keeping
The Manual must indicate that all records associated with the implementation of the Manual must be kept on the facility's premises for a period of ten years, and that all records must be made available to the CFIA upon request.
C. Identification of All Process Steps Associated with the Regulated Articles
All processing steps and activities related to receiving and handling regulated articles must be described and documented in the Manual.
The Manual must clearly identify the nature of the regulated articles being received and handled by the facility.
The Manual must contain a general diagram of the overall flow of articles and by-products (wash water, liquid and solid wastes, culls, peels, other potato parts, soil, used containers, etc.) throughout the facility, from receiving to shipping. The diagram should primarily focus on articles, installations and activities associated with receiving, handling, processing and disposing of the regulated articles.
By-products generated by the facility, such as rejected potatoes, culls, potato parts, soil, wash water and used containers are considered regulated articles and must be subject to specific collection and disposal instructions in the Manual as part of all processing steps. Appendix 8 outlines additional disposal requirements.
Specific to every processing step, the identification of equipment, areas, procedures, responsible staff members, the use of logs and the creation of a layout and/or flow diagram may be required in the Manual for clarification.
C1. Receiving and storing the regulated articles
The Manual must identify the period of the year when the facility plans to receive the regulated articles, including an evaluation of the volumes to be received and the origin of the articles. All documents related to sourcing and purchasing the regulated articles must be made available to the CFIA upon request. All loads of regulated articles received by the facility must be recorded in a log. A template of the reception log must be included in an appendix to the Manual. This log must include the following information for each load of regulated articles:
- Reception date.
- Description of the articles.
- Quantity received.
- Load identification or tracking number, including a copy of the delivery bill.
- Packaging status (i.e. bulk or packaged), including package size.
- Cleanliness status (i.e. received as washed or not).
- Origin of the articles, including the certificate of origin, Phytosanitary Certificate, or Movement Certificate, if applicable.
- Status of load: accepted or rejected.
- Cleanliness of trailer after unloading.
- Notify the local CFIA contact person prior to or within one business day upon arrival of the first load of regulated articles.
C1.1 Description of the unloading area
A detailed diagram of the layout of the unloading areas must be appended to the Manual, including a description of the article flow, the washing/cleaning areas, and the type of surfaces onto which the regulated articles and the associated conveyance will be traveling and unloading (i.e. cement, gravel, dirt, etc.)
C1.2 Description of the unloading process
The Manual must contain the following information:
- A list of unloading equipment (trailers, conveyor belts, etc.)
- An unloading procedure.
C1.3 Managing rejected loads
The Manual must indicate a procedure for the disposal of rejected loads of regulated articles. The CFIA must be contacted for a Movement Certificate if a rejected load is going to a destination other than the one stated in their Manual. All rejected loads must be recorded in a log. A template of the rejection log must be included in an appendix to the Manual. This log must include the following information for each rejected load:
- Load identification or tracking number, including a copy of the delivery bill.
- The delivery date.
- Reasons for rejection.
- Movement Certificate number if applicable.
- Final destination.
C1.4 Storing
The Manual must contain the following information:
- A procedure for identifying and operating the storages that ensures the regulated articles are segregated from non-regulated articles. All movement of regulated articles within storage must be recorded in a log. A template of the storage log must be included in an appendix to the Manual. This log must track all regulated articles in and out of the storage (e.g., bins, regulated potatoes, etc.)
- A detailed diagram of the layout of the storage area to be appended to the Manual.
C2. Grading
The Manual must contain the following information:
- A list of grading equipment.
- A grading procedure.
C3. Washing and brushing
The Manual must contain the following information:
- A list of washing and brushing equipment.
- Washing and brushing procedures.
C4. Packing and repacking
The Manual must contain the following information:
- A list of packing and repacking equipment.
- Packing and repacking procedures.
C5. Peeling
The Manual must contain the following information:
- A list of peeling equipment.
- Peeling procedures.
C6. Cooking
The Manual must contain the following information:
- A list of cooking equipment.
- Cooking procedures.
C7. Sprout inhibition
The Manual must contain the following information:
- A list of sprout inhibition equipment.
- Sprout inhibition procedures. Sprout inhibition activities must be recorded in a log. A template of the sprout inhibition log must be included in an appendix to the Manual. This log must contain:
- the load/or lot identification,
- the sprout inhibition products used,
- the application rate,
- the date and time of treatment,
- the name of the person who performed the treatment.
Should the sprout inhibition be performed prior to receiving the regulated potatoes, treatment information must be collected and records must be kept in association with the above log or independently. This includes treatment procedures as stated above, and any documents ensuring the identification and traceability of the sprout-inhibited lots.
C8. Other Processing Steps
The Manual must describe any other processes applied by the facility to the regulated articles. The Manual must indicate all mitigation measures implemented in order to reduce the phytosanitary risks associated with these other processes.
C9. Cleanup Activities, including Collecting and Disposing of Regulated Articles and By-Products
The Manual must describe cleanup activities including the collection and disposal of regulated articles and by-products generated at every processing step.
This includes a description of all procedures used for cleaning equipment and areas dedicated to unloading, storing, conveying, grading, washing and brushing, packing and repacking, peeling, cooking, sprout inhibition, or any other processing steps.
This also includes a description of all procedures used for collecting and disposing of all waste associated with the regulated articles being handled, processed or treated, such as rejected potatoes, culls, potato parts, soil, wash water, and containers and packages used to store and transport the regulated articles.
In addition, the Manual must contain a description of any other cleanup activities of equipment or areas, as well as any procedures used to ensure segregation of the regulated articles and possible by-products. This includes end-of-season cleanup activities for facilities not handling regulated articles year-round, and transitioning to a period when non-regulated articles will be received or handled.
Refer to Appendix 8 and PI-016 for more details.
C10. Final Collection and Disposal of Regulated Articles and By-Products
Final collection and disposal of regulated articles and by-products can occur on the approved facility's premises or using third party service providers (please see Appendix 8).
The Manual must clearly indicate how the facility will handle final collection and disposal of any regulated articles and by-products generated from the above processing steps (wash water, liquid and solid wastes, rejected potatoes, culls, peels, other potato parts, soil, used containers, etc.), including any other things potentially contaminated by contact with the regulated articles.
For the purpose of providing detailed instructions for final collection and disposal of by-products, the identification of equipment, areas, procedures, responsible staff members, the use of logs and the creation of a layout and/or flow diagram are required and must be identified in the Manual. All documents associated with the disposal of regulated articles and by-products including information such as destination, authorization, service providers, date, time, quantity, description of articles and by-products, bills of lading, conveyance, treatment, etc., must be collected and kept on file.
Appendix 8 specifies additional collection and disposal requirements.
Appendix 8: Guidelines for the Transport and Disposal of Regulated Articles and Cleaning Requirements
The overall principle addressing the final disposal of regulated articles and by-products exiting the approved facility is outlined in the CFIA's directive D-96-05, section 2.9.5. The purpose of this appendix is to provide additional instructions pertinent to disposal options and cleaning. Guidance on what to include in the facilities Manual regarding cleaning and disposal can be found in Appendix 7 section C9 and C10 of this document.
It is the approved facility's responsibility to ensure that additional local disposal requirements are met. This includes and is not limited to environmental considerations in municipal bylaws and regional, provincial and federal legislations.
A list of disposal sites to be utilized must be included in the facilities Manual. All the specifications (records, procedures, diagrams etc.) regarding the movement and the disposal of regulated articles must be defined in the Manual. In all cases, disposal procedures and sites will be evaluated and approved by the CFIA.
The CFIA may be required to issue regulatory documents such as Notice of Prohibition of Movement (CFIA/ACIA 0113) and/or Movement Certificates (MC) to control the movement and the disposal of regulated articles and by-products. If a MC is issued, all conditions on the issued document must be followed. In addition, all conditions and limitations applied to a disposal site must be clearly identified.
The requirements pertaining to the transport and disposal of regulated articles apply to:
- Movement of regulated articles
- Cleaning sites
- Commercial and private landfills sites
- Other disposal sites (such as retention lagoons and treatment facilities)
Note: It will be at the discretion of the regional CFIA office to determine if an inspection of the disposal facility is required.
A. Movement of regulated articles
All means of transporting regulated articles must be leak-proof. The transporting vehicle, trailer or container is to be cleaned in an appropriate manner prior to the next load at an approved cleaning site. This applies to both the transport of regulated articles from the United States (U.S.) and within Canada.
B. Cleaning sites
The cleaning site location can either be one of the following:
- Importing/receiving facility:
- Vehicles, containers or mini totes containing or transporting regulated articles from the U.S. to Canada or from a regulated area in Canada can be cleaned directly at the importing or receiving facility.
- Third party location
- In some circumstances, it may be more convenient to use a different cleaning site than the importing or receiving facility.
C. Commercial and private landfills sites
The site has been granted a current valid permit or license from the appropriate municipal, regional and/or provincial authorities to operate or has been recognized as satisfactory by CFIA to contain any potential pests of concern.
The landfill site must have a clearly defined boundary and location (Global Positioning System (GPS) coordinates may be used).
The regulated articles and by-products presented for disposal must be entirely covered within a one-day period by a layer of any suitable covering material that will efficiently prevent the regulated articles from being exposed to the environment. The covering material could consist of domestic garbage, compacted soil or other standard covering materials. The final covering must be permanent and stable meaning that it should not be removed or moved, unless it has been discussed with CFIA and CFIA has provided written authorization.
The CFIA may authorize the disposal of regulated articles back to the place of origin. For disposal in regulated areas within Canada, provisions of deep burial may not apply. Please contact your local CFIA person for disposal options.
D. Other disposal sites (such as retention lagoons or treatment facilities)
Floating material and sediments should be contained and disposed of as solid waste.
Segregated clear water should be discharged into an approved collecting and treatment and/or disposal system (e.g. a municipal sewage treatment system).
Liquid waste from retention lagoons cannot be used to irrigate agricultural land. Lagoons (natural or constructed) must be designed to prevent possible overflow.
The regulated articles generated following the emptying and/or the cleaning of a lagoon or treatment system must be disposed of in compliance with the disposal requirements outlined in this document.
For disposal sites within Canada, CFIA may be required to place additional regulatory restrictions on these sites.
General cleaning guidelines for processing and packing equipment
Each facility will have its own individual cleaning procedure which will be part of their Manual.
Some points to consider:
- The dismantling of equipment for effective cleaning may have to occur.
- After clean up, all equipment and the facility itself must be free of soil.
- Closed piping that cannot be disassembled must be considered (i.e., may need to be flushed first before non-regulated articles/loads can be accepted through this system).
- Bulk boxes comprised of wooden material may be difficult to clean effectively.
- Conveyor belts may need to be taken apart to clean effectively.
- The washing of transport vehicles and containers will have to occur every time they leave a facility. Unless otherwise stated in the Manual and authorized by the CFIA.
- The equipment of the processing and packing lines may only be cleaned once a week or a major clean up at the end of the CA period based on the use/reception intensity of the regulated articles.
- Roll-off and roll-on containers (which strictly carry only waste to disposal sites) will only be required to be cleaned at the end of the CA period.
For more information on cleaning standards please refer to PI-016