Purpose
The purpose of this document is to provide general guidance to applicants on the registration status/triggers for fertilizers and supplements regulated under the Fertilizer Act.
Registration under the Fertilizers Act
All fertilizers and supplements when sold or imported into Canada are regulated under the federal Fertilizers Act and regulations. Pursuant to these regulations, the products must be safe with respect to plant, animal, human health and the environment, and properly labelled to ensure safe and appropriate use.
A fertilizer is defined in the Fertilizers Act as any substance or mixture of substances, containing nitrogen, phosphorus, potassium or other plant food, manufactured, sold or represented for use as a plant nutrient
A supplement is defined as any substance or mixture of substances, other than a fertilizer, that is manufactured, sold or represented for use in the improvement of the physical condition of soils or to aid plant growth or crop yields
Some fertilizers and most supplements are subject to mandatory pre-market assessment and registration by the Canadian Food Inspection Agency (CFIA) prior to importation and sale in Canada.
As part of product assessments, CFIA officials evaluate the safety information/data and review the label to verify product compliance with the act, regulations and prescribed standards. Please note that all ingredients in the product (both active and inert) as well as the potential contaminants and degradation products are considered when reviewing product safety.
All regulated products, including those requiring registration and those exempt from registration, must meet prescribed standards for safety and labelling before they can be imported or sold in Canada. In addition to pre-market assessment and registration, product compliance is verified by CFIA area staff through inspections, product sampling and analysis, and marketplace label verification. Non-compliant products are subject to enforcement actions which may include product detention, and in cases of severe and/or repeated non-compliance, prosecution.
Determining the regulatory status of a product under the Fertilizers Act and regulations largely depends on how the product is represented in the marketplace. This includes nutrient sources, guarantees and/or product grades. All fertilizers and supplements imported into or sold in Canada require registration unless they meet one or more of the exemptions provided for in the Fertilizers Regulations.
Fertilizers exempt from registration
The following fertilizers are exempt from registration (except seeds or growing media)
- (a) a fertilizer that does not contain:
- a substance produced by or derived from a living organism
- a pesticide
- a supplement that is not registered and is not set out in the List of Primary Fertilizer and Supplement Materials
- a registered supplement, if the directions for use of the product are not consistent with those of the registered supplement
- a micronutrient fertilizer that is not registered, and
- a registered micronutrient fertilizer, if the directions for use of the product are not consistent with those of the registered micronutrient fertilizer
- (b) a fertilizer that is set out in the List of Materials
- (c) a customer-formula fertilizer and
- (d) a fertilizer whose active ingredient consists solely of a mixture of fertilizers or fertilizers and supplements, if those fertilizers and supplements are exempt from registration or are registered for the proposed use of the mixture.
Supplements exempt from registration
The following supplements are exempt from registration, (except seeds or growing media):
- (a) a supplement set out in the List of Materials and
- (b) a supplement whose active ingredients consist solely of supplements, if
- each supplement in the mixture is exempt from registration or is registered for the proposed use of the mixture and
- in the case of a mixture containing one or more viable microorganisms as active ingredients, the mixture is not further cultured or manipulated
Seed and growing media
Seeds that are treated with fertilizers, supplements, or both are exempt from registration if each of those fertilizers or supplements is either
- Exempt from registration
- Registered for that specific use
Growing media may include potting soils, liquid media, coco coir, wood fibers, peat moss, vermiculite, clay, pumice, and perlite
A growing medium that contains fertilizers or supplements or both is exempt from registration if each of those fertilizers or supplements is either exempt from registration or registered for that use, and is consistent with the direction for use of that media.
Triggers flow-charts
Consult the diagrams below to determine if your product is exempt from registration. If you are still not sure if a product is exempt from registration after consulting the diagram, you can submit an inquiry (IQ) to the CFIA.
The inquiry service is available to assist companies in understanding regulatory requirements and to give product-specific guidance on the type of information/data that will be required to support product registration. Applicants should consult the checklist of information required for an IQ to ensure that the CFIA has the information needed to provide an accurate and timely response. An evaluator will review the submitted information and provide a comprehensive response within 30 working days of receipt of the inquiry, as per published Service Delivery Standards. There is no charge for the inquiry process.
If a product does not meet an exemption, registration must be obtained before the product can be legally sold or imported in the Canadian marketplace.
The sale and importation of a non-compliant fertilizer or supplement product is considered a contravention under the Fertilizers Act, and may be subject to enforcement action.
Registration requirements for fertilizers
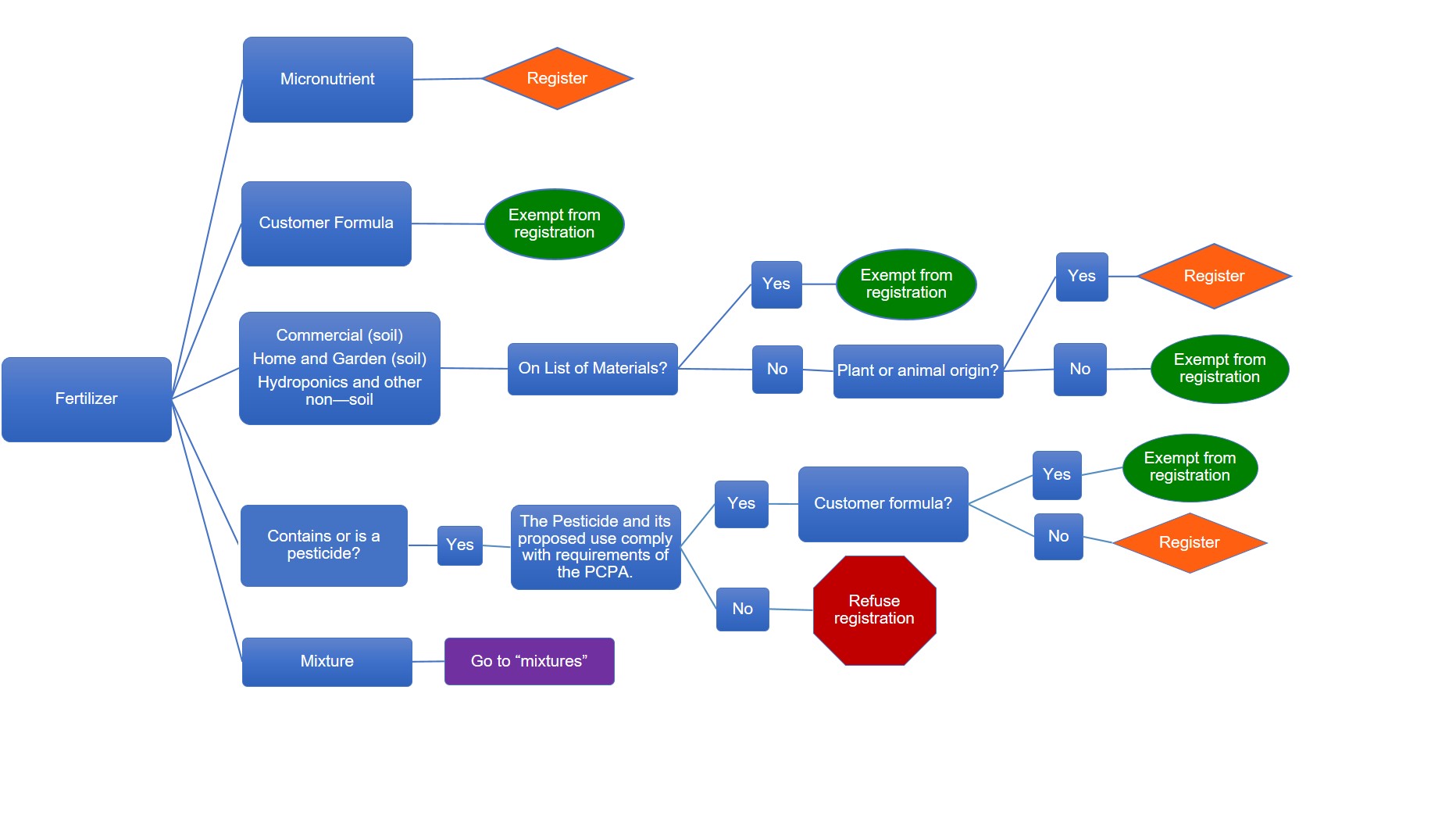
Description of flowchart – Registration requirements for fertilizers
The table below outlines when a fertilizer must be registered as shown in the flowchart image above
| Fertilizer type | On list of primary fertilizer and supplement materials | Origin | The pesticide and its proposed use comply with requirements of the Pest Control Products Act (PCPA) | Formula | Register or exempt |
|---|---|---|---|---|---|
| Micronutrient | n/a | n/a | n/a | n/a | Register |
| Customer formula | n/a | n/a | n/a | n/a | Exempt |
| Commercial (soil) Home and Garden (soil) Hydroponics and other (non – soil) |
yes | n/a | n/a | n/a | Exempt |
| Commercial (soil) Home and Garden (soil) Hydroponics and other (non – soil) |
no | plant or animal origin | n/a | n/a | Register |
| Commercial (soil) Home and Garden (soil) Hydroponics and other (non – soil) |
no | not plant or animal origin | n/a | n/a | Exempt |
| Contains or is a pesticide | n/a | n/a | yes | Customer formula | Exempt |
| Contains or is a pesticide | n/a | n/a | yes | Not customer formula | Register |
| Contains or is a pesticide | n/a | n/a | no | n/a | Refuse registration |
| Mixture | n/a | n/a | n/a | n/a | Go to "mixtures" |
Registration requirements for supplements
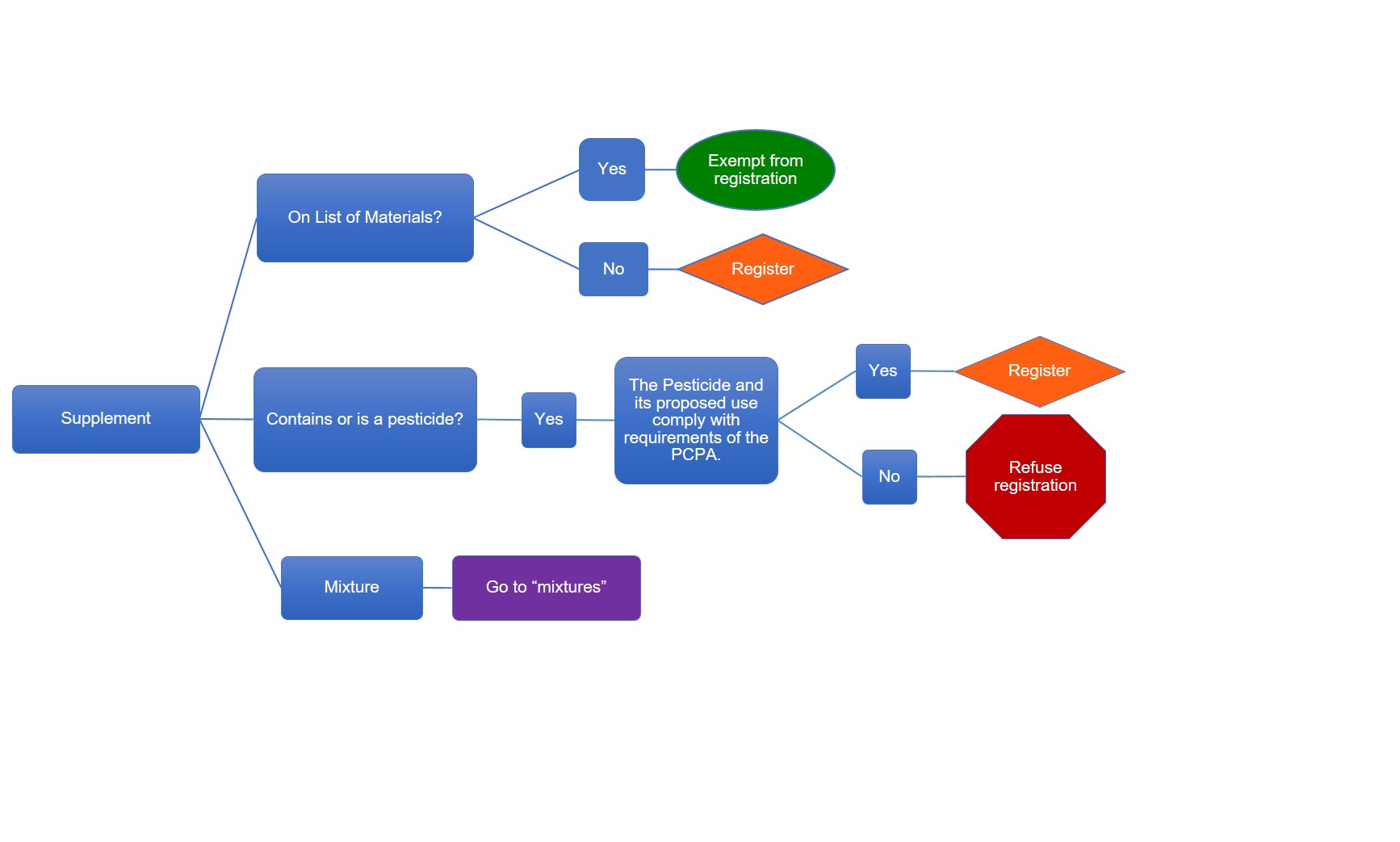
Description of flowchart – Registration requirements for supplements
The table below outlines when a supplement must be registered as shown in the flowchart image above
| On list of materials | Contains or is a pesticide | The pesticide and its proposed use comply with requirements of the Pest Control Products Act | Register of exempt |
|---|---|---|---|
| Yes | n/a | n/a | Exempt |
| No | n/a | n/a | Register |
| n/a | Yes | Yes | Register |
| n/a | Yes | No | Refuse registration |
For mixtures, please go to: "Registration requirements for mixtures".
Registration requirements for mixtures
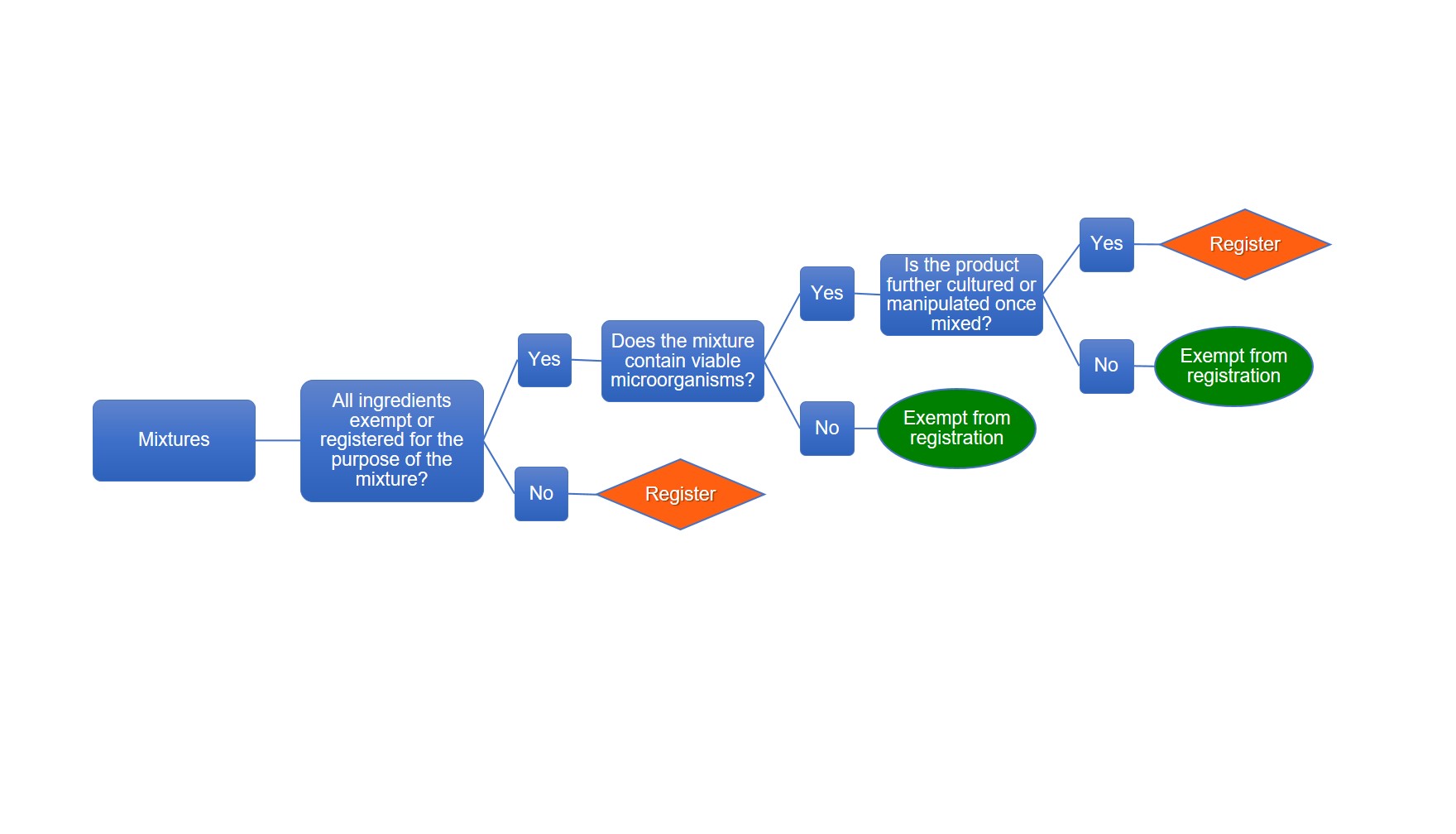
Description of flowchart – Registration requirements for mixtures
The table below outlines when a mixture must be registered as shown in the flowchart image above
| Ingredients | The mixture contain viable microorganisms | The product is further cultured or manipulated once mixed | Register or exempt |
|---|---|---|---|
| All ingredients exempt or registered for the purpose of the mixture | yes | yes | Register |
| All ingredients exempt or registered for the purpose of the mixture | yes | no | Exempt |
| All ingredients exempt or registered for the purpose of the mixture | no | n/a | Exempt |
| All ingredients are not exempt or are not registered for the purpose of the mixture | n/a | n/a | Register |
Registration requirements for products containing seeds
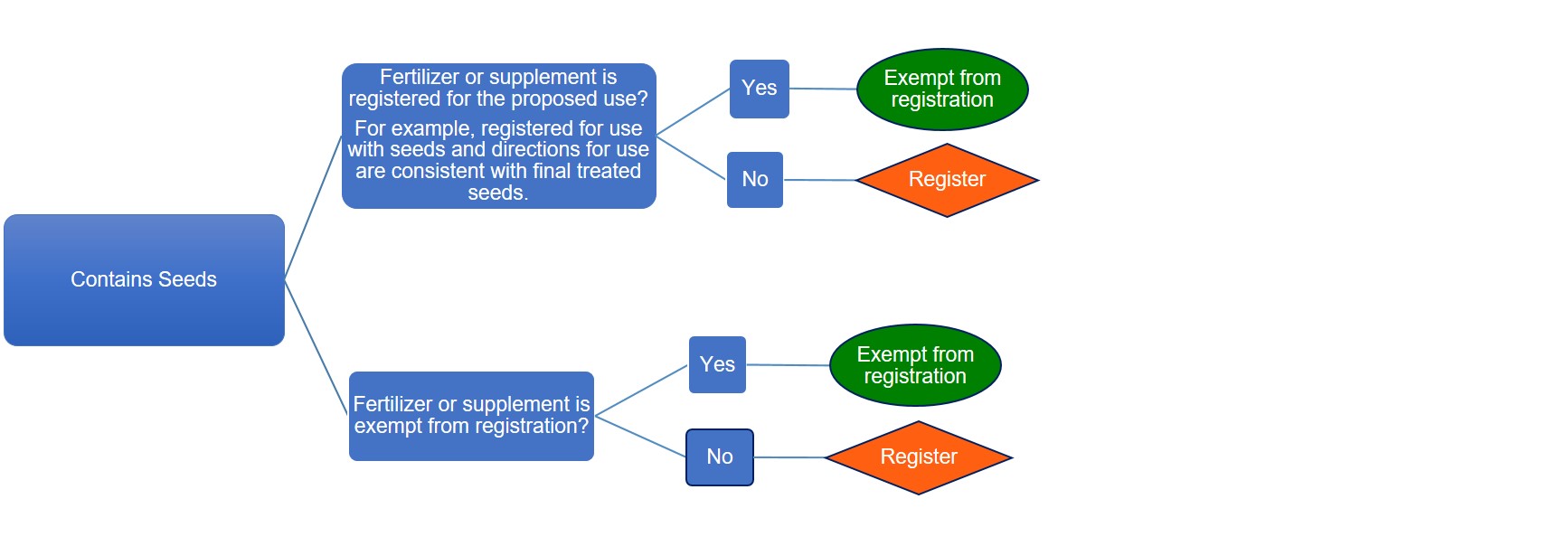
Description of flowchart – Registration requirements for products containing seeds
The flowchart shows when fertilizers or supplements containing seeds require registration:
- Fertilizer or supplement that is registered for the proposed use for example registered for use with seeds and directions for use are consistent with final treated seeds, is exempt from registration
- Fertilizer or supplement that is registered but not for the proposed use for example not registered for use with seeds and directions for use are not consistent with final treated seeds, must be registered
- Fertilizer or supplement that is exempt from registration and contains seeds, is exempt from registration
- Fertilizer or supplement that is not exempt from registration and contains seeds, must be registered
Registration requirements for products containing growing media
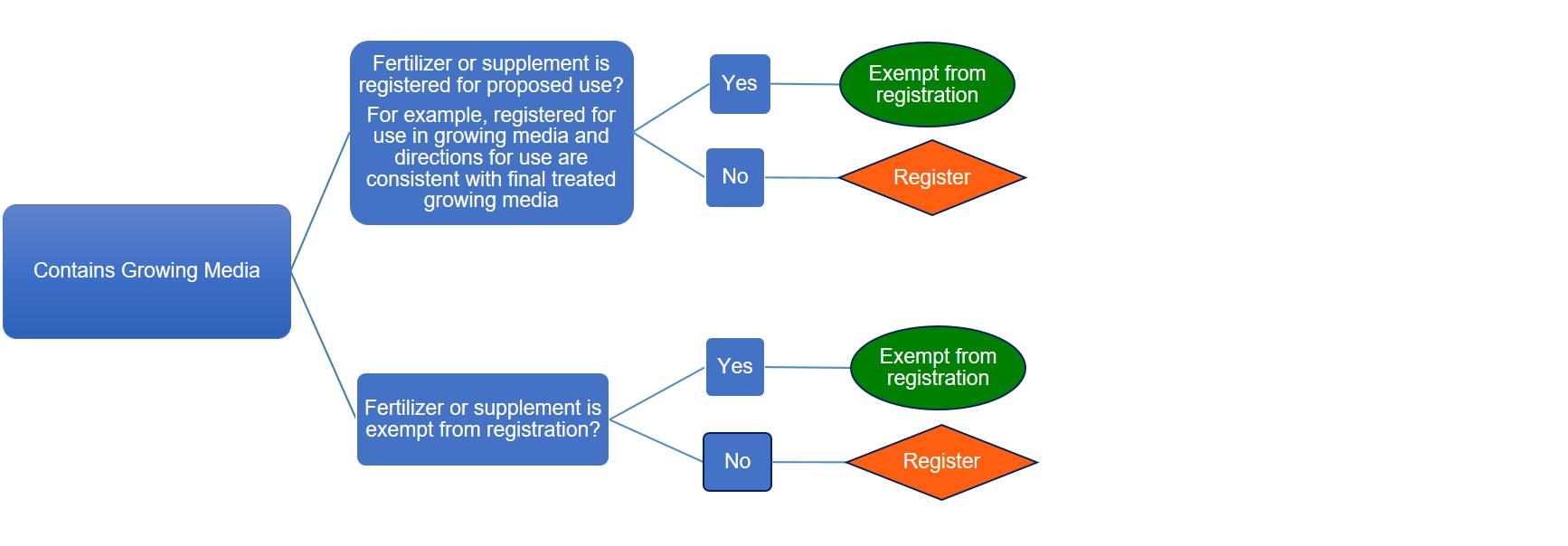
Description of flowchart – Registration requirements for products containing growing media
The flowchart shows when fertilizers or supplements containing growing media require registration:
- Fertilizer or supplement that is registered for the proposed use for example registered for use in growing media and directions for use are consistent with final treated growing media, is exempt from registration
- Fertilizer or supplement that is registered but not for the proposed use for example not registered for use in growing media and directions for use are not consistent with final treated growing media, must be registered
- Fertilizer or supplement that is exempt from registration and contains growing media, is exempt from registration
- Fertilizer or supplement that is not exempt from registration and contains growing media, must be registered
Contact information
Fertilizer Safety Section
c/o Pre-market Application Submissions Office (PASO)
Canadian Food Inspection Agency
Phone: 1-855-212-7695
Email: cfia.paso-bpdpm.acia@inspection.gc.ca