On this page
- 1.0 Purpose
- 2.0 Authorities
- 3.0 Reference documents
- 4.0 Definitions
- 5.0 Acronyms
- 6.0 Operational procedure
- 7.0 Appendix
1.0 Purpose
The purpose of this document is to provide guidance to Canadian Food Inspection Agency (CFIA) inspection staff when conducting a food ingredient verification.
The procedure outlined below may be used when verifying compliance of a list of ingredient on a food label, to support export certification, to aid in the assessment of a Preventive Control (PC) related sub-element, as part of a food safety investigation or follow-up to a complaint.
This guidance is written with the assumption that inspection staff have been trained in the Standard Inspection Process (SIP) and in ingredient verification techniques.
This document is intended to be used in conjunction with CFIA guidance as referenced in section 3.0, reference documents.
2.0 Authorities
- Agriculture and Agri-Food Administrative Monetary Penalties Act
- Canadian Food Inspection Agency Act
- Food and Drugs Act (FDA) (as it relates to food)
- Food and Drug Regulations (FDR) (as it relates to food)
- Safe Food for Canadians Act (SFCA)
- Safe Food for Canadians Regulations (SFCR)
The inspection powers, control actions and enforcement actions authorized by the above legislation are identified and explained in the Operational guideline – Food regulatory response guidelines.
3.0 Reference documents
- Operational Guidance: Standard Inspection Process
- Grades and Standards: documents Incorporated By Reference (Under SFCR)
- Operational Guideline: Categorizing labelling and advertising non-compliance and timeframes for correction in food (accessible only on the Government of Canada network – RDIMS 9912657)
- Operational guidance: Food sample collection
- Operational Guideline – Food regulatory response guidelines
- Food preventive control and traceability inspection – System verification
- CFIA Sampling Information (accessible only on the Government of Canada network)
- Ingredient Verification Worksheet (accessible only on the Government of Canada network– RDIMS 12776151)
- DSDP SOP INS – conducting an inspection (accessible only on the Government of Canada network – RDIMS 9839405)
- Industry Guidance – Dairy processing systems: Vitamin Addition
- Industry Guidance – CFIA Industry Labelling Tool
- Health Canada – Templates for label designers: nutrition facts table and list of ingredients
- Health Canada – Lists of Permitted Food Additives
- Health Canada – Marketing Authorizations
4.0 Definitions
Definitions are located in the documents listed below or as a defined word where it is intended to supersede the definitions within the glossary documents:
- Fortification
- A process by which vitamins, mineral nutrients and amino acids are added to foods to provide consumers with sufficient but not excessive amounts of certain nutrients in their diet. Fortification may be mandatory or voluntary depending on the food product. The requirements for fortification are set out in Part B and Part D, Division 3 of the FDR. (Enrichissement)
- Ingredient verification inspection
- An activity that assesses the compliance of a food product with the Food and Drugs Act and Regulations (FDAR) and the Safe Food for Canadians Act and Regulations (SFCAR) in respect to its composition, ingredients, allergen labelling and advertising statements and claims. The activity involves an inspection at a regulated party's premises to assess the accuracy, completeness and truthfulness of these statements and claims. (Inspection pour la vérification des ingrédients)
- Manufacturing Floor recipe (also known as a Make Sheet)
- The form employees complete when they prepare a batch of food product. (Fiche de fabrication)
- Master formula
- The master formula is the manufacturer's recipe, which provides the identity and quantity of each ingredient used to prepare the product. (Formule originale)
- Product specification sheet (commonly referred to as a spec. sheet)
- An information sheet that documents all the attributes and information regarding the food in question. (Fiche de spécification)
5.0 Acronyms
Acronyms are spelled out the first time they are used and are consolidated in the Food business line acronyms list.
6.0 Operational procedure
This operational procedure provides inspection staff guidance when conducting a food ingredient verification.
Where more specific guidance is required then what is provided in the Standard inspection process (SIP), these will be indicated in this section.
Commodity inspection operational guidance (OG) refers the inspector to the SIP for basic guidance on the 4 inspection steps. If the commodity inspection is being conducted to support a preventive control inspection (PCI) currently underway, some or parts of the inspection steps will have already been completed.
6.1 Prepare for inspection
Refer to SIP, section 3.0, step 1 - Prepare for the inspection. In addition to the general guidance provided in SIP, the following applies.
| DSDP field | DSDP data field selection |
|---|---|
| Inspection trigger | Preventive Control Inspection Plan Sample Collection Plan Commodity Inspection Plan Incident Response Domestic Permission Export Permission Import Permission |
| Task type | Commodity inspection |
| Inspection task level 1 | Inspect Commodity |
| Inspection task level 2 | Ingredient verification |
6.2 Conduct the inspection
Refer to SIP, section 4.0, step 2 – Conduct the inspection. In addition to the general guidance provided in the SIP, the following applies.
6.2.1 Selecting a product for the inspection
Prioritize your product selection by targeting a product:
- with a confirmed or suspected non-conformity (for example: as a result of a complaint, identified during other inspection activities);
- which a label verification is also being performed or
- that is new or has a new label,
6.2.2 Conduct a preliminary label verification
Conduct a preliminary label verification to determine which regulatory requirements apply for the specific product. In particular, confirm:
- If the product requires a list of ingredients,
- If there are conditions that would allow for an exemption from displaying the list of ingredients from any regulations.
The labelling requirements checklist available in the Industry Labelling Tool (ILT) may be used as a guide for the preliminary label verification.
6.2.3 Ingredient verification
The steps to conducting an ingredient verification are the same regardless of where the verification is taking place but the documentation available may vary depending on if the regulated party is a SFC license holder or not.
When conducting an ingredient verification, the Ingredient Verification Worksheet (accessible only on the Government of Canada network – RDIMS 12776151) is available, if needed.
6.2.3.1 Inspection procedure at the manufacturer level
- Obtain the current finished product label with a list of ingredients. It can be a copy of the label obtained from the manufacturer or if a product sample is collected at the same time, that label can be either kept or a picture can be taken of it.
- Obtain the master formula that lists the name and quantity of each ingredient that makes up the final composition of the end product.
- Record the ingredients in descending order of proportion by weight, before they are combined to make the final food in the worksheet or on a separate sheet. If the formula indicates certain ingredients by volume, convert these volumes to weight to determine the proper descending order of the ingredients.
- Confirm the accuracy of the master formula by viewing the actual ingredients used. Review all the components of the ingredients. This information may be found in the ingredient listed on the container or from the manufacturer's product specification sheet(s). Record the actual ingredients used and their components.
- Review the ingredient packages and specifications sheets for any other useful information.
- For example, brand name, name and address declared on the label
- Specification sheets should identify the raw materials and any substitutions in the raw materials.
- Where appropriate, manufacturers may substitute, vary or omit certain ingredients from their formula based on availability, pricing, etc., as per B.01.011 of the FDR. Record these potential variations, if any are identified in the formula.
- Observe a batch of product as it is being prepared to confirm the actual amount of each ingredient added. If this is not possible, use the company's manufacturing floor recipe from a previous production run.
- Observe the handling and storage conditions for any of the priority allergens that may be present in the facility and identify any areas of possible cross-contamination.
- Assess the ingredients for compliance with the compositional requirements prescribed for the food by the FDR and SFCR.
- Ingredients are foods on their own and must meet the respective compositional requirements established in the FDR and other applicable legislation. Therefore, components of ingredients must be permitted in the ingredient to which they are added. However, components of an ingredient do not need to be permitted in the final food. For example, in the case of a cake mix made with liquid whole eggs and where the liquid whole eggs contain nisine (additive). The additive is permitted in liquid whole eggs but not in the cake mix (final product). Since it is not added to the cake mix but to the ingredient of the cake mix (the liquid whole eggs) then this complies.
- If the ingredient(s) added to the product are not used as prescribed, verify if Health Canada issued a Marketing Authorization (MA) to allow a food not in compliance with the regulations to be marketed while an amendment to permit its on-going legal sale is being processed. Refer to the List of Marketing Authorizations found on Health Canada's website.
- Verify the list of ingredients and the master formula to determine whether vitamins, minerals or amino acids have been added for fortification
- If yes, then consult the table following section D.03.002 of the FDR or the table "Foods to Which Vitamins, Mineral Nutrients and Amino Acids May or Must be Added" under the Nutrient Content Claims section of the ILT in conjunction with the appropriate sections of the FDR (Part B and sections D.01.009-D.01.011, D.02.009) to determine whether the food contains nutrients in the specified amounts.
- If the nutrients added to the food are not used in compliance with the regulations, check whether Health Canada has issued any Marketing Authorization (MA). Refer to the List of Marketing Authorizations found on Health Canada's website.
- Compare the current finished product label to the master formula and the information gathered on the ingredients. Verify that:
- All ingredients and components that make up the finished product are declared in the list of ingredients except those exempted by the FDR. Each ingredient and component must be declared by its common name.
- The ingredients are permitted in the finished product, and that they are present at permitted levels. As needed, see Appendix 1 for the conversion of additives.
- The ingredients from the master formula appear in descending order by weight before they are combined to form the food [FDR B.01.008.2(3)]. Some ingredients can be declared in any order at the end of the list:
- spices
- seasonings
- herbs (except salt)
- flavours
- flavour enhancers
- vitamins and their salts
- mineral nutrients and their salts
- food additives.
- Priority allergens and gluten are declared as required by the FDR B.01.010.1(2); and sulphites are declared as required by the FDR B.01.010.2(3). More information can be found in the List of Ingredients and Allergens section of the ILT
- Sugars based ingredients are grouped under "Sugars" [B.01.008.3(1), FDR].
- Assess the ingredients listed for compliance with the compositional requirements prescribed for the finished food product by the FDR and the SFCR. If the ingredient(s), added to the product are not used as prescribed, verify if:
- CFIA granted a Test Market Authorization (TMA) to the company for the use of the ingredient(s) in their specific product. Ask the company if they have obtained a TMA.
- Health Canada issued a Marketing Authorization (MA) to allow a food not in compliance with the regulations to be marketed while an amendment to permit its on-going legal sale is being processed. Refer to the List of Marketing Authorizations found on Health Canada's website.
- Determine if the French list of ingredients on the label corresponds to the English list. The order in which ingredients are listed must be the same in both languages. However, since some ingredients may be listed in any order at the end of the list, as explained in above, those ingredients should be declared in the same order in both languages to avoid confusion.
6.2.3.2 Inspection procedures – Other than manufacturing level
The availability of the master formula will depend on whether the party is a SFC license holder or not. When a product is examined at the location of a SFC license holder, documents providing evidence that measures are effective (for example, the master formula) must be available under their Preventive Control Plan (PCP). If documentation is not available, expanding the scope may be necessary. Discuss the issue with your supervisor and refer to the SIP and Food preventive control and traceability inspection – System verification.
Where the regulated party is not a license holder, the master formula may not be readily available. In order to complete the ingredient verification, the inspector may:
- Request the regulated party to obtain more information from the manufacturer,
- Review the list of ingredients declared for any obvious non-conformities, and/or
- Submit products for laboratory analysis when needed (consult the CFIA Sample Information (accessible only on the Government of Canada network) and the Operational Guideline: Food sample collection).
- Request traceability documents
6.2.4 Categorize non-compliance
If a non-compliance is identified related to food labelling and advertising, consult the Operational Guideline: Categorizing labelling and advertising non-compliance and timeframes for correction in food (accessible only on the Government of Canada network – RDIMS 9912657(link doesn't change only the text)) and SIP, section 4.7.
Consult the Operational guideline: food regulatory response guidelines if any product action is deemed necessary (for example: seizure and detention) and SIP section 4.5 to determine if immediate control action is required.
6.2.5 Capturing notes related to commodity inspection in the DSDP
For information on capturing notes relating to commodity inspections in the DSDP, refer to Appendix A section 5.4.1 of the SIP and section 3.5.1 of the DSDP SOP INS – conducting an inspection (accessible only on the Government of Canada network – RDIMS 9839405).
In addition to capturing an accurate description (brand name, common name, net quantity, lot number) of the commodity inspected in the "Commodity Description" field in DSDP, enter the same commodity description in the "objective evidence" field along with the non-compliances found so that it appears in the final inspection report.
Save a copy of the assessed label and the Ingredient Verification Worksheet if used to conduct the ingredient verification in RDIMS and reference the RDIMS number in the "documents" field of the DSDP.
6.3 Communicate the results
Refer to SIP, section 5.0, step 3 – Communicate the inspection results.
6.4 Conduct follow-up
Refer to SIP, section 6.0, step 4 – Conduct the follow-up inspection.
For general inquiries related to this operational guidance document, follow established communication channels, including submitting an electronic Request for Action Form (e-RAF)-(accessible only on the Government of Canada network).
7.0 Appendix
Appendix 1: Converting food additives in terms of reference chemicals
Some of the food additive tables in Health Canada's List of Permitted Food Additives prescribe the maximum level of use for certain additives in terms of reference chemicals rather than the actual food additive used. In these cases, you must use molecular weights to convert the levels permitted for the reference chemical to the level permitted for the actual additive being used.
When the maximum level permitted is expressed in parts per million (p.p.m.), the mathematical formula is:
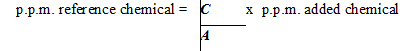
Where: "C" is the molecular weight of the reference chemical (Refer to Table 2); "A" is the molecular weight of the added chemical (Refer to Table 2), and; "p.p.m. added chemical" is the level calculated from the product formulation.
In some situation you may also have to convert the amount of the additive used into parts per million (ppm). It may be easier to understand the concept of parts per million (ppm) if we consider that one ppm is one part out of one million parts. For example, one gram in one million grams or one millilitre out of one million millilitres. It is also commonly understood that one ppm is equal to 1 mg of solute in 1 L of solution.
For the sake of comparison, 1 ppm is equivalent to:
- 1 mg solute / L solution
- 1 mg solute / 1 000 mL solution
- 0.001 g solute/ L solution
- 0.000001 kg solute / L solution
- 4.546 mg de solute / gal solution
- 3.785 mg solute / US gal solution
Example of calculation to convert food additives in terms of reference chemicals
According to list 11, part 2 item S.10 and S.3 of the list of permitted additives of Health Canada, the maximum level of sodium metabisulphite permitted in frozen sliced apples is 500 p.p.m., calculated as sulphur dioxide. The manufacturer's recipe indicates that 300 g sodium metabisulphite is added to frozen sliced apples and the final yield of frozen sliced apples is 250 kg. [Assuming no moisture loss.]
In order to determine if the amount of sodium metabisulphite added is acceptable:
First, convert the 300 g (300,000 mg) of sodium metabisulphite to parts per million of sodium metabisulphite added to the final food:

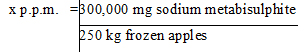
x = 1200 p.p.m. of sodium metabisulphite is added to the frozen apples
Then, convert of sodium metabisulphite to sulphur dioxide:
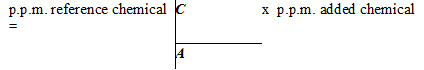
C, the molecular weight of sulphur dioxide, is 64.1 g/mol as indicated in Table 2;
A, the molecular weight of sodium metabisulphite, is 190.1 g/mol as indicated in Table 2, and; 1200 p.p.m. sodium metabisulphite is added.
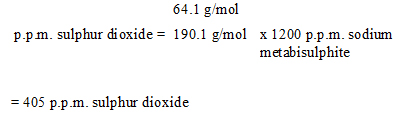
The calculated concentration of sulphur dioxide, 405 p.p.m., is less than 500 p.p.m., therefore the amount of sodium metabisulphite added to the recipe is satisfactory.
When the maximum level permitted is expressed on a percentage basis, the mathematical formula is:

Where: "C" is the molecular weight of the reference chemical; "A" is the molecular weight of the added chemical, and; "% added chemical" is the level calculated from the product formulation.
| Additive | Molecular weight of added chemical (g/mol) | Calculated as | Moleculaar weight of reference chemical (g/mol) | Refer to the following HC list for the maximum level of use |
|---|---|---|---|---|
| Caffeine citrate | 386.3 | Caffeine | 194.2 | 8. List of Permitted Food Additives with Other Accepted Uses, Item C.2 |
|
Calcium chloride:
|
|
Calcium | 40.1 | 6 List of Permitted Firming Agents, item C.1 |
|
Calcium citrate:
|
|
Calcium | 40.1 | 6 List of Permitted Firming Agents, item C.2 |
| Calcium disodium ethylenediaminetetraacetate (calcium disodium EDTA) (tetrahydrate) | 446.3 | Anhydrous calcium disodium EDTA | 374.3 | 12. List of Permitted Sequestering Agents, item C.2 |
|
Calcium lactate:
|
|
Calcium | 40.1 | 6. List of Permitted Firming Agents, item C.3A |
|
Calcium phosphate monobasic:
|
|
Calcium | 40.1 | 6. List of Permitted Firming Agents, item C.5 |
| Calcium propionate | 186.2 | Propionic acid | 74.1 | 11. List of Permitted Preservatives (Part 3), Item C.1 |
|
Calcium sulphate:
|
|
Calcium | 40.1 | 6. List of Permitted Firming Agents, item C.6 |
|
Methyl-p-hydroxybenzoate |
152.1 | p-Hydroxybenzoic acid | 142.1 | 11. List of Permitted Preservatives (Part 2), Item M.1 |
| Potassium benzoate (trihydrate) | 214.3 | Benzoic acid | 122.1 | 11. List of Permitted Preservatives (Part 2), item P.1 |
| Potassium bisulphite | 121.2 | Sulphur dioxide | 64.1 | 11. List of Permitted Preservatives (Part 2), item P.2 |
| Potassium ferrocyanide, trihydrate | 422.4 | Anhydrous sodium ferrocyanide | 303.9 | 1. List of Permitted Anticaking Agents, Item P.1.1 |
| Potassium metabisulphite | 222.3 | Sulphur dioxide | 64.1 | 11. List of Permitted Preservatives (Part 2), item P.3 |
| Potassium phosphate dibasic | 174.2 | Sodium phosphate dibasic | 160.0 | 12. List of Permitted Sequestering Agents, Item P.4 |
|
Potassium pyrophosphate |
330.3 | Sodium phosphate dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item P.5 and 12. List of Permitted Sequestering Agents, Item P.3 |
| Potassium tripolyphosphate | 448.4 | Sodium phosphate dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item P.5.2 |
|
Propyl-p-hydroxybenzoate |
180.2 | p-Hydroxybenzoic acid | 142.1 | 11. List of Permitted Preservatives (Part 2), item P.5 |
| Sodium acid pyrophosphate | 221.9 | Sodium phosphate dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item S.1.1 |
| Sodium benzoate | 144.1 | Benzoic acid | 122.1 | 11. List of Permitted Preservatives (Part 2), item S.1 |
| Sodium bisulphite | 140.1 | Sulphur dioxide | 64.1 | 11. List of Permitted Preservatives (Part 2), item S.2 |
| Sodium dithionite | 174.1 | Sulphur dioxide | 64.1 | 11. List of Permitted Preservatives (Part 2), item S.8 |
| Sodium ferrocyanide, decahydrate | 484.1 | Anhydrous sodium ferrocyanide | 303.9 | 1. List of Permitted Anticaking Agents, item S.3 and 8. List of Permitted Food Additives with Other Accepted Uses, Item S.5 |
| Sodium hexametaphosphate | 611.8 | Sodium phosphate dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item S.6 |
| Sodium metabisulphite | 190.1 | Sulphur dioxide | 64.1 | 11. List of Permitted Preservatives (Part 2), item S.3 |
| Sodium potassium tripolyphosphate | 752.3 | Sodium phosphate, dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item S.7.3 |
| Sodium propionate | 96.1 | Propionic acid | 74.1 | 11. List of Permitted Preservatives (Part 2), Item S.3.1 and (Part 3), Item S.2 |
|
Sodium pyrophosphate |
265.9 |
Sodium phosphate, dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item S.7.4 |
| Sodium salt of methyl-p-hydroxy benzoic acid | 174.1 | Methyl-p-hydroxy benzoate | 152.1 | 11. List of Permitted Preservatives (Part 2), item S.4 |
| Sodium salt of propyl-p-hydroxy benzoic acid | 202.2 | Propyl-p-hydroxy benzoate | 180.2 | 11. List of Permitted Preservatives (Part 2), item S.5 |
| Sodium sulphite | 126.1 | Sulphur dioxide | 64.1 | 2. List of Permitted Bleaching, Maturing or Dough Conditioning Agents, Item S.3 and 11. List of Permitted Preservatives (Part 2), item S.7 |
| Sodium tripolyphosphate | 367.9 | Sodium phosphate, dibasic | 160.0 | 8. List of Permitted Food Additives with Other Accepted Uses, Item S.11 |
|
Stannous chloride:
|
|
Tin | 118.7 | 8. List of Permitted Food Additives with Other Accepted Uses, item S.13 |
| Sulphurous acid | 82.1 | Sulphur dioxide | 64.1 | 11. List of Permitted Preservatives (Part 2), item S.10 |