On this page
- Abbreviations and special terms used in the report
- Executive summary
- 1. Introduction
- 2. Assessment objective, scope, and methodology
- 3. Legal basis for the assessment
- 4. Background
- 5. Government authorities, roles and responsibilities
-
6. The CRRS
- 6.1 Background
- 6.2 Overview of the program
- 6.3 Auxiliary parties involved in the implementation of the CRRS
- 6.4 Resources and training of parties involved in the implementation of the CRRS
- 6.5 Recognition/authorization and oversight of auxiliary parties
- 6.6 Implementation of food safety controls at the farm, packing and exporter levels
- 7. Laboratory capacity
- 8. Closing meeting
- Appendix A. The government organizations responsible for the CRRS
- Appendix B. Overview of the CRRS Process (steps and parties involved)
- Appendix C. Summary of SENASICA's action plans/comments to the CFIA's recommendations from the Canadian Food Inspection Agency On-site Assessment Report of Mexico's Food Safety Control System for Fresh Fruit and Vegetables – 2017
Abbreviations and special terms used in the report
- AAO
- Authorization and Approval of Individual and Legal Entities Office of DIAOOPA
- CA
- Competent Authority
- CAs
- Corrective Actions
- CFIA
- Canadian Food Inspection Agency
- CB
- Collaboration Body
- COFEPRIS
- The Federal Commission for the Protection Against Sanitary Risks
- CRO
- Certification and Recognition Office of DIAOOPA
- CRRS
- Contamination Risk Reduction System
- DIAOOPA
- Directorate of Agri-Food Safety, Organic Operation and Pesticides for Agricultural Use
- FFV
- Fresh Fruits and Vegetables
- FSC
- Food Safety Coordinator
- FST
- Food Safety Technician
- GAPs
- Good Agricultural Practices
- ISO
- International Organization for Standardization
- NRCPC
- National Reference Center for Pesticides and Contaminants
- SAGARPA
- Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food
- SENASICA
- National Service of Health, Food Safety and Agro-Alimentary Quality
- SRRC
- Contamination Risk Reduction System
- TPP
- Authorized Third Party Professional (Specialist)
- TPS
- Third Party Specialists
Executive summary
This report summarizes observations made during the Canadian Food Inspection Agency's (CFIA) March 2017 on-site assessment of Mexico's government oversight of food safety controls for fresh fruits and vegetables (FFV).
The main objective of the visit was to increase CFIA's understanding of Mexico's food safety system as it relates to FFV, that is the Contamination Risk Reduction System (CRRS), and to verify its implementation. It was also intended to strengthen our relationship with the competent authority (CA) of Mexico (National Service of Health, Food Safety and Agro-Alimentary Quality (SENASICA)) and, to increase their awareness of Canadian import requirements for FFV.
The results of this assessment will inform the CFIA's import controls and help the CFIA direct oversight resources to areas of highest risk. This can lead to increased consumer confidence in imported products, while contributing to market access benefits.
The assessment was conducted March 6 to 17, 2017 at sites in 7 states and included: meetings with the CA, 12 primary production units (farms and packing facilities), 5 Collaboration Bodies (CBs) and the National Reference Center for Pesticides and Contaminants (NRCPC).
The key elements of the assessment included:
Authorities
- Current food legislative authorities, including regulations, standards, codes of practice, and arrangements
- Authority to recognize and accredit parties involved in the CRRS
- Authority to carry out microbiological risk assessments, monitoring and surveillance activities
- Authority to respond to non-compliances where risk has been identified, for example, recalls, other control and enforcement activities
Government organization and resources
- The roles and responsibilities of the various government departments and authorities involved in the CRRS
- The resources, responsibilities, functions, and coordination between the parties involved in the CRRS
- Resources and competencies of the CA parties involved in the delivery of the CRRS
- Analytical support facilities and programs, for example, number of laboratories, facilities and equipment, accreditation, monitoring programs, etc.
Third parties involved in the implementation of the CRRS
- Organizations responsible for the implementation of the CRRS and their relationship with the CA
Inspection, enforcement and surveillance activities
- Role of the CA in inspection, surveillance, and enforcement
The assessment determined that the CA has established a voluntary system for the reduction of contamination risks for FFV (the CRRS). This program ensures that FFVs are produced under optimal sanitary conditions.
Under the current legislative structure, Mexico does not have the authority to require mandatory participation in the CRRS for either export or domestic markets. However, if the CA has an agreement with an importing country that requires specific products to be produced under the CRRS, then the CRRS becomes mandatory. For example, this is the case with the United States of America (for papayas, cantaloupes and cilantro) and Japan (for avocadoes).
This report provides a number of recommendations which highlight opportunities for improvement and enhancement of the CA's implementation of the CRRS.
The observations and recommendations contained in this report are based on information provided to the assessment team through the Canadian Assessment Standards tool, personal interviews, and on-site observation. They represent the collective understanding of the members of the assessment team.
1. Introduction
An assessment was conducted in Mexico March 6 to 17, 2017, by a team from the CFIA's Food Import/Export Division, under the auspices of the Food Safety Oversight initiative. The twelve-day mission included:
- interviews with Mexico's CA and third parties involved in the implementation of the CRRS
- visits to both conventional and organic facilities involved in the primary production, packaging, storage and export of various FFV (melons, papayas, mango, bananas, strawberries, tomatoes, green onions, cilantro, fresh herbs and cucumbers) – including fields, shade-houses and macro tunnel operations
- a visit to the National Reference Centre laboratory involved in the testing of fresh produce
An opening meeting was held with the CA on March 6, 2017, during which the objectives of the visit were reviewed, technical aspects of the assessment plan and the itinerary were confirmed. During this meeting the CA provided an overview of their roles and responsibilities. The team also had an opportunity to review various documents and further clarify their understanding of the CRRS.
From March 7 to 16, 2017 the team visited production and packing units recognised/in the process of becoming recognised under the CRRS, various third parties involved in the delivery of the CRRS (CBs, Authorized Third Party Professionals (TPPs) and Third Party Specialists (TPSs)), and the NRCPC. Representatives of the CA and the CFIA Technical Specialist from the Canadian Embassy in Mexico accompanied the assessment team throughout the visit.
A closing meeting was held on March 17, 2017 to summarize the observations of the team, including opportunities to further strengthen the CRRS.
2. Assessment objective, scope, and methodology
The key objectives of the assessment were to:
- confirm our understanding of the roles, responsibilities, and authorities of the CA in the oversight of food safety as it relates to the CRRS
- observe the implementation of the CRRS
- increase awareness of Canadian import requirements and to observe how the controls applied by Mexico's fresh produce industry meet these requirements
- discuss the status of the ongoing assessment of Mexico's Organic system for the purpose of negotiating an Organic equivalency arrangement
2.1 Methodology
The assessment was comprised of 3 phases.
Phase I – A desk review of information provided to the CFIA through Mexico's response to the Canadian Assessment Standard tool.
Phase II – An on-site visit to observe the implementation of the CRRS.
Phase III – Drafting of the assessment report to summarize the assessment team's understanding of the CRRS and to highlight opportunities to further strengthen the system.
2.2 Assessment scope summary
The visit focused on primary producers and packers of fresh produce who were recognised/were in the process of being recognised under the CRRS. The number and variety of units visited provided a representative sample of commodities, diversity in size, complexity, and geographical location to allow the team to draw an unbiased conclusion about the implementation of the system as a whole. In addition, the assessment team met with the CA, several CBs and the NRCPC as outlined in Table 1.
| Site visited | No of sites visited | Location |
|---|---|---|
| CA | 1 | Mexico City |
| Primary production units | 10 | Various locations |
| Packing units/exporters Table Note 1 | 8 | Various locations |
| CBs | 5 | Various locations |
| Laboratories | 1 | Tecamac, state of Mexico |
Table Notes
- Table Note 1
-
Some operations had both farms and packing facilities at the same location. In addition, some operations had more than 1 farm and/or packing facility at the same location. A total of 12 locations were visited.
3. Legal basis for the assessment
This assessment was conducted in agreement with the Mexican CA and under the CFIA's Import Requirements for Fresh Fruits and Vegetables outlined in Section 3.1 (1) (b) of the Fresh Fruit and Vegetable Regulations and Section 4 of the Food and Drugs Act. In particular:
-
Section 3.1 (1) (b) of the Fresh Fruit and Vegetable Regulations:
"Subject to subsection (2), no person shall market in import, export or interprovincial trade as food unless it is not contaminated."
-
Section 4.(1) of the Food and Drugs Act:
"No person shall sell an article of food that
- has in or on it any poisonous or harmful substance;
- is unfit for human consumption;
- consists in whole or in part of any filthy, putrid, disgusting, rotten, decomposed or diseased animal or vegetable substance;
- is adulterated; or
- was manufactured, prepared, preserved, packaged or stored under unsanitary conditions."
4. Background
Mexico's fresh fruit and vegetable industry consists of operations that range in size, complexity, and capacity. Production units range in size from farms of less than 2 acres operations with several hundred acres (which may include several farm properties and/or packing facilities). Some of the larger operations offer accommodation, health and social services, schools, etc. for their employees. All locations observed have basic services (electricity, running water, etc.).
Fresh produce is typically imported into Canada from Mexico either directly or via the United States of America. The main FFV products Canada imports from Mexico include tomatoes, peppers, berries and avocadoes Mexico-Canada Trade Report January, 2017.
Fresh produce imported from Mexico has previously been linked to foodborne illnesses in Canada involving pathogens such as Salmonella species, and Cyclospora cayetanensis. Products implicated included fresh herbs, green onions, papayas and melons. As a result, several products have been recalled by the CFIA.
In response to multiple Canadian outbreaks of Salmonella species which were linked to cantaloupe from Mexico, the CFIA engaged Mexico's CA to develop and implement preventative upstream control measures including monitoring food safety practices of primary production and packing facilities in Mexico. In the meantime, the CFIA put in place an import policy for cantaloupes from Mexico which recognizes Mexico's requirement for cantaloupe exporters to operate under the CRRS. The policy references SENASICA's published list of Mexican companies approved to export cantaloupes to Canada.
5. Government authorities, roles and responsibilities
The Federal Plant Health Law of Mexico provides the regulatory authority and jurisdiction for the CRRS (Articles 1, 2, 3, 7-A, 47-A to 47-J, see Appendix B). Under this law, the Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA) has the authority to:
- establish measures for the risk reduction of contamination in the primary production of vegetables, necessary to minimize the presence of physical, chemical, and microbiological contamination determined through an hazard analysis
- issue Official Standards, other legal provisions related to the CRRS, and technical documents based on Good Agriculture Practices (GAPs)
- carry out evaluations, verifications, audit and certifications of farms, packing houses, places, establishments or facilities related to primary production. Such evaluations or audits could be carried out with the initiative of the Ministry of Agriculture or by an individual or legal entity request
- organize and operate the certification, inspection and monitoring of the primary production processes of the plants, where GAPs are applied
- recognize third party professionals and specialists in order to help the Ministry in the implementation and surveillance of the compliance of GAPs in the farms
- approve, with prior accreditation and under specific topics, to individual and legal entities for operating as: certification bodies, verification units or laboratories
5.1 Roles and responsibilities
SENASICA's General Directorate for Agri-food Safety, Aquaculture and Fisheries is responsible for the implementation and oversight of the CRRS. The program is delivered by a section of the General Directorate for Agri-food Safety, Aquaculture and Fisheries called the Directorate of Agri-Food Safety, Organic Operation and Pesticides for Agricultural Use (DIAOOPA).
The DIAOOPA is comprised of 5 offices with defined roles and responsibilities for the implementation of the CRRS (see Appendix A). These are the offices of:
- Certification and Recognition
- Federal Verification and Inspection
- Authorization and Approval of Individual and Legal Entities
- Food Safety Programs, Regulatory and International Affairs
- Monitoring and Surveillance of Contaminants and Toxic Waste
The responsibilities of these offices are:
Certification and Recognition Office of DIAOOPA (CRO)
- Verify corrective actions (CAs) submitted by operators and TPSs as part of the recognition process under the CRRS (both initial and ongoing)
- Asses audit reports prepared by TPSs on the implementation of the requirements of the CRRS
- Issue letters of recognition to operators who successfully implement the CRRS
- Suspend or revoke certificates of recognition and issue administrative monetary penalties for the violation of the provisions of this act
Federal Verification and Inspection Office of DIAOOPA
- Plan and execute the National Inspection Program of individual and legal entities, farms, and areas recognized for implementing the CRRS in agro-alimentary products, livestock, fisheries, organic production and the commercial houses that sell pesticides
Authorization and Approval of Individual and Legal Entities Office of DIAOOPA (AAO)
- Authorize individuals and legal entities who are interested in serving as third parties in the evaluation of compliance with the CRRS, for example, TPPs, TPSs, and testing laboratories
- Conduct performance evaluation (oversight) of the third part entities, that is, TPPs, TPSs, and laboratories
Food Safety Programs, Regulatory and International Affairs (that is the Safety Programs, Regulatory and International Affairs Office of DIAOOPA)
- Assess the performance of the CBs
Monitoring and Surveillance of Contaminants and Toxic Waste Office of DIAOOPA
- Coordinate and implement the National Program for Monitoring and Surveillance of Contaminants and Toxic Waste in the primary production of food in order to identify the hazards in agro-alimentary products, livestock and fisheries
- Supervise and execute the development of the National Program for Monitoring and Surveillance of Contaminants and Toxic Waste in local or foreign products, to prevent them from exceeding the maximum permissible levels
6. The CRRS
6.1 Background
The first set of food safety laws for FFV was published following the creation of SENASICA in 2001. This led to the development of a food safety program in 2003 under which the first recognition was issued to an avocado producer in 2005. The program served as the foundation for the CRRS which came into being in 2013 with the publication of the legal framework to establish food safety programs. The purpose of the framework was to build a productive agriculture and fisheries sector, strengthen food safety and health of the population, and to increase the competitiveness of the agricultural sector.
Although the system is largely a voluntary oneFootnote 2, it has grown to 7,263 recognized operators in the primary production of fruits and vegetables (2016). SENASICA encourages the industry to participate in the program as a way of ensuring that they meet the import requirements of other countries (for example, The US Food Safety Modernization Act, etc.).
SENASICA is developing a new regulation in order to make it mandatory. Some existing modules from the current CRRS will be incorporated in the new regulation.
6.2 Overview of the program
The CRRS is based on GAPs and Good Manufacturing Practices (Hazard Analysis Critical Control Point principles). It is comprised of 15 core modules:
- Registration
- Infrastructure
- Hygiene
- Domestic and Wild Fauna Management
- Training and Skills Development
- Internal Evaluation
- Procedure Validation
- Traceability
- Production History
- Water Management
- Fertilization
- Good Use and Management of Agrochemicals
- Harvest
- Packaging
- Transport
The phases and activities in the CRRS recognition process include:
- Diagnosis
- Know the production conditions in which the hazard assessment and the food safety technical plan is developed.
- Planning
- Implementation of the CRRS requirements
- Initial internal audit
- Operators interested in obtaining CRRS recognition identify a person who will be responsible for food safety (Food Safety Technician; FST) who has appropriate training and/or experience.
- As a first step, the operator engages a TPP (who may be associated with a CB or, an independent contractor) to assist them in meeting the requirements of the program and, to prepare them for a third party audit of their system.
- A diagnosis (pre) audit is conducted while the operator is in the process of implementing the requirements of the CRRS to determine the level of readiness to proceed in the process.
- Initial application for recognition
- Granting of a control number
- Once the operator is ready, they submit a request to SENASICA's CRO which includes all of the documentation to support their application. SENASICA assigns them a control (identification) number.
- Granting of a control number
- Evaluation
- External audit (by a TPS)
- Review of CAs
- A third party audit is conducted by a TPS to verify that the company has met the requirements of the CRRS. The TPS communicates their audit findings to the operator at the closing meeting and, captures them in an audit report. The report is sent to the CRO for evaluation. The CRO notifies the operator that they have 45 days to address non conformities by providing a corrective action plan. Operators must submit their corrective action plan to the CRO along with evidence of CAs taken for their assessment and verification.
- Both the pre-audit and the third party audit are conducted using a common checklist which was developed by SENASICA.
- Recognition (valid for 2 years)
- Decision to recognize
- Publication on SENASICA's website (Spanish only)
- The CRO evaluates the CAs implemented by the operator as a result of the third party audit. If the operator is determined to meet the requirements of the program, a certificate of recognition is issued and the operator becomes part of the CRRS. The company is then listed on SENASICA's website (Spanish only).
- Maintenance
- Internal audit (maintenance)
- Random inspection by SENASICA
- Once an operator has been recognized, they are subject to an annual internal audit, conducted by a TPP who is different from the TPP who helped the operator to implement the system. Non conformities are issued by the TPP. A copy of the internal audit report is sent to the CRO for evaluation to maintain recognition. The same approach is taken to addressing non-conformities as for third party audits.
- SENASICA also conducts random inspections of operators recognized under the CRRS to verify ongoing compliance.
- Re-application for recognition (every 2 years) and renewal
- Every 2 years the operator must complete the same phases as the initial process in order to renew their recognition.
6.3 Auxiliary parties involved in the implementation of the CRRS
Although the CRRS is a government program, its delivery is dependent on the work of various third party entities that are authorized by SENASICA to conduct the work. These include:
CBs
CBs are organizations of producers who support SENASICA in the development and delivery of phytosanitary and CRRS measures. There is 1 CB in each of Mexico's 32 states.
Each CB has a Technical Manager, a Food Safety Coordinator (FSC), several TPPs and Technical Assistants who support the delivery of the CRRS in each state.
Funding for the CBs comes from both SENASICA and the state government.
The main activities of the CBs and their TPPs are to:
- provide advice to operators on how to comply with the CRRS
- train operators and their staff on GAPs and Good Manufacturing Practices
- take samples
- conduct internal audits
- provide equipment and other physical support, for example, signage, portable washrooms, personal protective equipment, etc. (if financing is available)
Authorized third party professionals independents
These are TPPs who are not employed by CBs. They work independently.
Third Party Specialists (TPSs)
TPSs are independent professionals who verify the implementation of the CRRS by operators.
As of February 2017, 333 TPPs and 75 TPSs were recognized in the system. A current list of recognized (auxiliary) personnel is available on SENASICA's website.
6.4 Resources and training of parties involved in the implementation of the CRRS
The delivery of the CRRS relies on resources from the following organizations who need to maintain their technical skills and credentials:
SENASICA staff who work in the implementation of the CRRS
There are 63 SENASICA officers involved in the implementation of the CRRS. They are required to have a degree in agronomy, veterinary science, chemistry, or biology. Once hired, they complete hands-on training by shadowing an experienced inspector during verifications and inspections. They then conduct their own verifications and inspections under the supervision of an experienced inspector until they are considered to be fully trained. They can then conduct the work independently.
In order to maintain their competencies, they are required to receive 40 hours of training on relevant topics each year. Training is delivered by universities, specialized organizations, and research institutes. Training records are maintained. In addition, every officer undergoes an annual performance evaluation as per the requirements of the Professional Career Service Law on Federal Public Health.
Authorized third parties (TPPs, TPSs)
As of January, 2017 there were 333 authorized TPPs and 75 TPSs. Authorized third parties, for example, TPPs, TPSs must also have a degree in agronomic, biological, biochemical or agri-food-related sciences. They must demonstrate knowledge in topics such as GAPs, toxicology, good use and management of agrochemicals, Hazard Analysis Critical Control Point System, traceability and recalls, principles of microbiology, and sampling procedures and, they need to have at least 1 year of experience in a related field. TPSs are also required to have specific training in audit processes.
TPPs and TPSs are required to attend relevant training offered by SENASICA or external providers every year. FSCs maintain records of training received by TPPs and TPSs while the Manager of the CB maintains training received by the FSC.
6.5 Recognition/authorization and oversight of auxiliary parties
SENASICA conducts various activities under their authorities:
CBs
SENASICA oversees the CBs once they are granted recognition by SAGARPA. Through this recognition, SAGARPA delegates financial support and certain authorities for the delivery of the CRRS and other programs (aquaculture, etc.). The recognition is valid for 2 years.
The CBs are subject to both document and on-site reviews by the Safety Programs, Regulatory and International Affairs Office of DIAOOPA. Annual plans are prepared by each CB. They are reviewed and authorized by SENASICA before funding is provided.
The CBs are subject to both document and on-site reviews by SENASICA. Each month, SENASICA reviews the financial reports of each CB to ensure that program spending is on track. In addition, the CB submits an annual report on their activities to SENASICA for their review. This is complemented by on-site assessments (every 2 years) of the delivery of the program activities using a checklist which was developed by SENASICA. In this way, SENASICA can confirm that the CBs are delivering their mandate and, that enrolment in the system is increasing. SENASICA issues a report of their assessment of each CB which includes observations and a deadline to address them. In general, the CB is given 10 days to address observations.
CBs have systems in place to prevent conflict of interest, for example, TPPs who inspect a farm/packing unit will not be assigned to audit that operator; TPPs are rotated to avoid getting too familiar with a site; TPPs who are related to, or are friends, with an operator will not be assigned to that operator, if possible.
TPPs and TPSs
Candidates apply to become a TPP or TPS by submitting a documentation package in to DGIAOOPA's AAO. The application package includes training records, a log of experience, and a conflict of interest declaration. Following successful review of the information submitted, authorization is granted by the AAO through an agreement between the 2 parties. It is valid for 2 years. Renewal is subject to re submission of the same application package.
Although both TPPs and TPSs are authorized by SENASCIA, their performance is overseen by different parties, as indicated in Table 2. For example, TPPs who are employed by CBs are monitored and assessed by the FSC of their CB, whereas TPSs are evaluated by the AAO.
With respect to TPPs who work with a CB: the approach to monitoring their work varies in type, frequency and type of documentation used for the assessment and, for documenting the outcome of the evaluation. For instance, some CBs monitor the work of their TPPs on a yearly basis, focusing on the number of visits and quality of their work, while other CBs conduct on-site visits at operators to observe the work of the TPP.
TPSs are subject to supervision and performance evaluation by SENASICA at any time. The evaluation of TPSs consists of document reviews and on-site observations. SENASICA plans to evaluate 10% of TPSs each year. Candidates are selected through an annual draw.
As part of the document review process, each TPS is required to submit a monthly report of their activities to the AAO. It includes dates of evaluations, products involved, duration of the audit, and observations made. The AAO assesses the reports to identify training needs, and if needed, modifies existing tools and templates.
In addition, the CRO reviews the quality of the third party audit reports generated by the TPSs. Any inconsistencies are shared with the DIAOOPA's Federal Inspection and Verification Office and the AAO for assessment as part of the ongoing monitoring and evaluation of the TPS. AAO can suspend or cancel authorization of a TPS as a result of their evaluation.
SENASICA requires that TPSs are rotated to ensure third party audits are conducted by a different TPS each time.
Laboratories
Laboratories can be directly accredited by the national accreditation body Entidad mexicana de acreditación, a.c (Mexico's national accreditation body) or, they can be authorized to conduct various analyses by SENASCIA. The process of accreditation and authorization are different, however, operators under CRRS must use a laboratory that meets International Organization for Standardization (ISO) 17025 for the testing of official samples.
In collaboration with the NRCPC, SENASICA's AAO authorizes private laboratories based on the requirements of ISO 17025. There are 11 authorized laboratories. They are subject to oversight by SENASICA in collaboration with the NRCPC.
| Party overseen | Oversight conducted by: |
|---|---|
| CBs | SENASICA (the Safety Programs, Regulatory and International Affairs Office of DIAOOPA) |
| FSC of each CB | Manager of the CB |
| TPPs associated with a CB | FSC of the CB |
| Independent TPPs | SENASICA – not observed |
| TPSs | SENASICA (AAO) |
| Private laboratories | SENASICA (AAO) in collaboration with the NRCPC |
6.6 Implementation of food safety controls at the farm, packing and exporter levels
All of the operations visited had implemented the CRRS and 1 of them was in the process of implementing it.
The assessment team focused on verifying the implementation of the following core requirements of the CRRS:
- general requirements and infrastructure
- hygiene
- training and skills development
- internal audits
- good harvesting practices
- traceability
- validation procedures and sampling
- records
- food safety plans
- Standard Operating Procedures
- flow diagrams
- other records to support the implementation of the CRRS
General observations
The CFIA team observed that operators were committed to the effective implementation of the CRRS and, that the companies visited had the general requirements and infrastructure in place to support the CRRS.
Each operator had a Manual of Procedures which detailed the hazard analysis, critical controls, process and employee flows, field and facility maps, roles and responsibilities, procedures, templates and other tools, training records, employee health and hygiene, and production-related elements of the CRRS.
All operations had a FST who is responsible for the implementation of the CRRS at their operation (which could include more than 1 farm and/or 1 or more packing facilities).
The FST coordinates several activities including the development, implementation and maintenance of the Manual of Procedures. The FSTs are supported by an appropriate number of staff which depends on the size of operation.
Employee entry procedures (hand washing, no jewellery or wounds, etc.) were clearly posted in strategic areas (entry point of the farm, packing facility, etc.) – in pictures and often, in multiple languages.
Operators had sanitation and hygiene controls in place in the fields and packing facilities.
Infrastructure was generally appropriate for the activities conducted and included systems in place to control exit and entry of workers, visitors, and wildlife.
CRRS requirements for water to be supplied from deep water wells and, to for access to be restricted to designated trained staff were met.
Pesticides and agro-chemicals were stored in secure areas accessible only by designated trained staff. Controls were in place for the disposal of empty agro-chemical containers. Agro-chemicals were handled only by trained staff.
Operators implemented annual surveillance plans and maintained detailed records for the analysis of water, workers' hands, surfaces, product, soil, etc. Plans varied between operators but generally included analysis of basic microbiological indicators (fecal coliforms, Salmonella species and Escherichia coli and chemical contaminants (heavy metals, pesticides, etc.). Samples were taken by CBs, external contractors, or an accredited laboratory contracted to conduct the analysis.
Although operators are required to maintain a sampling database on the indicator's behaviour of products, water sources and contact surfaces records were generally not maintained in a way that would facilitate trend analysis or investigation of an issue.
Employees were trained on general topics every year, for example, hygiene, internal procedures, good handling practices of agro-chemicals, use of personal protective equipment, etc. and on additional topics, for example, specific procedures as required. Training was provided TPPs, FSTs/their staff, SENASICA, and external training providers.
Employee hygiene was monitored and documented routinely, for example, at start up, after breaks, etc. Procedures are in place to remove employees if they don't meet the general health/hygiene requirements and, to retrain staff if appropriate.
Operators had implemented documented sanitation and pest control programs.
Operators implemented detailed traceability systems which allowed them to trace a product back to the farm and lot of origin, and in many cases, to the picker, packing line, implements used, etc.
All operators had documented traceability procedures. Mock recalls are conducted at least once a year to test the effectiveness of their plans. Records demonstrated that although the procedure is implemented, there are some areas for improvement to ensure that operators are able to respond to real life situations.
Although the operators visited were recognized/in the process of being recognized, not all of their suppliers were recognized. Nevertheless, measures were implemented in the packing houses to mitigate risk of contamination. For example, 1 packer sourced product from over 100 suppliers, only some of which were recognized under the CRRS.
Some operators were actively encouraging their suppliers to become recognized by funding training, etc. It is also our understanding that SENASICA is moving toward making core modules of the program mandatory for all operators of all sizes (small, medium, and large) and levels of trade (domestic, import and export).
All operators visited had CB-associated TPPs assigned to them to support their ongoing implementation of the CRRS. Some companies engaged an independent TPP to conduct additional internal audits to validate the work of their CB-associated TPP.
Each operator had been subject to: and internal audit (conducted by a TPP) and, a third party audit (conducted by a TPS), both of which were conducted using SENASICA's checklist.
Operators typically submitted evidence of CAs taken as a result of a third party audits to the CRO before they received CRO's request for CAs.
Only a few operators visited had been inspected by SENASICA's Federal Inspection and Verification Office for activities other than the special programs (cantaloupes, papayas, cilantro and avocadoes).
Most of the operations visited held third party certifications to various food safety schemes but these were not considered to be a factor in their recognition process.
Not all of the laboratories engaged by the operators visited were authorized directly by SENASICA. Instead, they were accredited by the Entidad mexicana de acreditación, a.c (Mexico's National Accreditation Body).
7. Laboratory capacity
The NRCPC (the laboratory) supports SENASICA in the detection and analysis of microbial and chemical contaminants in samples taken from the FFV sector.
The Centre has 4 sections: Microbial Contaminants, Chemical Contaminants, GMOs, and Monitoring and Quality Evaluation. These support 5 program areas:
- national program for monitoring of FFV operations
- program for monitoring contaminants in imported vegetables
- special programs (cantaloupes, papayas, avocadoes, cilantro) and sanitary alerts
- national program for monitoring fresh produce produced under the CRRS
- surveillance of organic products
The lab has 3 mobile laboratories which can be deployed to borders/ports to analyze FFV samples as an innovative approach to support trade.
General observations
The laboratory is accredited to ISO 17025 (9001 and 14000). It is also a member of several international associations and agreements.
The laboratory has a Quality Manager who is responsible for the Quality Manual which documents their methods and compliance with international standards as required by their various accreditations.
Staff are qualified and trained for their respective roles. Training is ongoing. It is conducted annually and, as required, for example, when new procedures are introduced.
The laboratory routinely participates in both national and international inter-laboratory comparison activities.
The laboratory receives samples from SENASICA's Federal Inspection and Verification Office and, from private clients. In both cases, if a pathogen/contaminant is detected, a process is in place to inform SENASICA (who would in turn inform the Department of Health's Federal Commission for the Protection Against Sanitary Risks (COFEPRIS) in event of a public health issue).
The laboratory analyzes an increasing number of samples each year. In 2016, the Centre tested more than 6000 samples compared to only 400 in 2013. In 2016, 310 samples were analyzed for chemical residues compared to 45 in 2013.
The laboratory continues to add new equipment and methods to extend both their scope and limits of detection to support the needs of their clients.
The laboratory has implemented internal methods for the detection of Cyclospora which are based on Polymerase Chain Reaction and microscopy. 100 samples were taken in 2016 following an issue with cilantro from Puebla.
The laboratory does not conduct analysis for viruses. This work is done by COFEPRIS.
The laboratory works with COFEPRIS to coordinate activities in event of a public health issue/outbreak related to FFV from Mexico.
The laboratory regularly exchanges technical information with COFEPRIS.
SENASICA engages the laboratory when they determine it is needed to support alerts/outbreak investigations.
Although there is no national procedure for the coordinated response to a foodborne disease outbreak, each department has their own procedures as per their authorities, and communications between departments are well established.
The laboratory has a national antimicrobial resistance program and a whole genome sequencing program. Together with COFEPRIS, the lab is creating a national geographical profile for the whole genome sequencing of Salmonella in support public safety and trade. They plan to expand the scope of this work to other pathogens in future.
8. Closing meeting
The closing meeting was held with SENASICA and representatives from the Canadian Embassy in Mexico on March 17, 2017. The CFIA team expressed their overall satisfaction with the oversight provided under SENASICA's CRRS program. A number of areas were highlighted where Mexico could further strengthen their program. An open discussion was held to give SENASICA an opportunity to clarify the team's observations and recommendations. This served to reinforce the good relations that the CFIA has with SENASCIA with respect to ensuring the safety of FFV exported to Canada.
8.1 Observations
During the closing meeting 23 observations were shared with the CA.
- Although the CRRS is voluntary (except in the case of specific Arrangements with importing countries), there is great commitment and dedication not only from SENASICA, but also from the auxiliary parties involved in the implementation of the CRRS.
- CRRS operations recognize the benefits of the program, and are working to bring their suppliers on board by promoting the food safety culture, funding training, etc.
- Without a specific agreement with an importing country, SENASICA does not have the authority to mandate the CRRS program for exports. Food safety oversight of exporters who are not recognized under the CRRS was not observed, but exports need to meet the requirements of the importing country.
- The roles and responsibilities within SENASICA's DIAOOPA are well defined and delegated.
- SENASICA staff has established good working relationships with the CBs, TPPs, TPSs, operators and laboratories.
- Auxiliary individuals involved in the implementation of the CRRS are well trained and are committed to the delivery of the system.
- The number of operators who are recognised under the CRRS increases each year, for example, 862 new operators were recognised in 2016.
- The recognition period for both operations and auxiliary parties (CBs, TPPs, TPSs and laboratories, etc.) is relatively short (2 years) and the current process of renewal is overly cumbersome and a burden on both applicant and SENASICA.
- SENASICA's recognition process does not appear to take into consideration previous recognition of the organization/individual by SENASICA or, third party certification, for example, Primus, Global Food Safety Initiative, the British Retail Consortium, etc.
- Although SENASICA is committed to conducting a number of oversight activities, they were unable to meet their goals for the monitoring of TPSs, and, reviewing CAs resulting from internal audits, third party audits and SENASICA inspections in a timely manner. SENASICA explained that this was a result of limited resources in 2016.
- There are no established criteria or frequency for conducting SENASICA inspections of recognized operators. They are currently determined by a random draw process. We understand that this process is being revised to focus activities on risk and triggers/complaints.
- SENASICA provides training to all organizations involved in the CRRS to bring consistency in the understanding of how to implement the program.
- The same checklist is used for different oversight activities conducted by, for example, TPPs, TPSs and SENASICA inspectors. This practice may limit the ability to thoroughly assess the program. In addition, since the assessment process is predictable, operators tend to focus only on the items in the checklist. This can limit their ability to think outside the scope of the checklist. As a result, they may not be fully prepared to respond to food safety issues.
- CBs (and their TPPs) play a critical role in promoting and implementing the CRRS by providing operators with ongoing technical assistance, training, and supplemental equipment (signage, etc.).
- CBs are supervised regularly by SENASICA through annual and monthly reports, as well as on-site visits.
- CBs do not apply a consistent approach to monitoring the work of the TPPs across the 32 states. For example, some CBs monitor the work of their TPPs on a yearly basis, focusing on the number of visits and quality of their work. Others CBs conduct on-site visits at operators to observe the work of the TPP.
- Although SENASCIA provides some training to TPPs and TPSs as part of their ongoing learning requirements, there is no national approach to training delivery to achieve consistency and maximize limited resources.
- Individual employee profiles are not maintained and training records are not documented to facilitate the identification of training requirements, for example, employee training files contain numerous certificates as opposed to a training plan with goals and achievements.
- Although there is no national procedure for the coordinated response to a foodborne disease outbreak, each department has their own procedures as per their authorities, and communications between departments are well established.
- Organic: SENASICA is currently adding additional requirements to their organic regulations that will require mandatory sampling. In addition, they are modifying some of the internal guidelines related to the activities of the Certification Bodies.
- SENASICA inspectors can take samples as part of their inspections of recognized operators, in response to complaints/alerts/outbreaks, and for special programs (cantaloupes, papayas, cilantro and avocadoes).
- SENASICA has a monitoring and surveillance program for chemical residues and microbiological contamination in domestic and imported FFV.
- SENASICA and COEFPRIS establish a collaborative base to define the actions and collaboration topics for both Agencies. With this collaborative base they establish and generate the management mechanisms to act during a sanitary risk related to the consumption of contaminated food, in order to give an immediate response, organized and planned in order to prevent risks related to consumer's health.
8.2 Gaps
- It was not clear how TPPs who work independently are overseen by SENASICA.
- Due to resource constraints, SENASICA was not able to deliver the planned number on-site assessments of TPSs in 2016 (10%).
8.3 Recommendations
The CFIA assessment team offered 11 recommendations which could further strengthen the CRRS.
- Revise the 2 year recognition process in order to reduce the administrative burden on all parties and re-allocate funds to areas of higher risk.
- Review the current processes for the authorization and monitoring of auxiliary parties (TPPs, TPSs, laboratories) to take into consideration the previous history of the individuals/ organizations and consider the potential value of third party certifications, for example, Primus, Global Food Safety Initiative, and the British Retail Consortium. The revised authorization process should focus on results of oversight activities, training received in the past 2 years, and any certifications granted.
- Consider developing a national CB network to identify best practices, share training resources and other information/experience to ensure consistent national delivery of the program.
- Consider developing a standardized template for the training and assessment of TPPs and TPSs as well as for recording and tracking employee training to facilitate the identification of training requirements.
- Re-assess the need for the ongoing presence of a TPP in the implementation of the CRRS once an operation has been recognized.
- Review SENASICA's CRRS oversight process that verifies the requirement for operators to develop and implement a 'database on the indicator's behaviour of products, water sources and contact surfaces' to ensure that operators understand what is expected of them and, they have the capacity to conduct trend analysis, etc.
- Review the oversight process for evaluating operator's traceability procedures to ensure that they are well-prepared to respond to real-life issues. For example, mock recalls should include players such as SENSASICA, CBs, etc. and CAs.
- Re-consider SENASICA's role in assessing CAs that are generated by TPSs, that is, conformities that they have not directly observed.
- Consider developing new outcome-based tools/checklists to asses conduct internal audits, third party audits and SENASICA inspections. This will allow the different parties involved to observe the operation from different perspectives, leading to a more comprehensive assessment of their implementation of the CRRS. This should also better prepare operators to respond to food safety issues.
- Evaluate the needs for additional program resources in order to meet increasing demands as more companies join the program.
- Review the current prioritization process to allocate resources to activities that are truly critical to the success of the program.
8.4 Conclusions and next steps
As a result of this visit, the CFIA assessment team has established a general understanding of Mexico's CRRS, and a solid foundation for good working relations with SENASICA.
SENASICA acknowledged the observations and recommendations presented by the CFIA team and expressed their future interest in collaboration.
Appendix A. The government organizations responsible for the CRRS
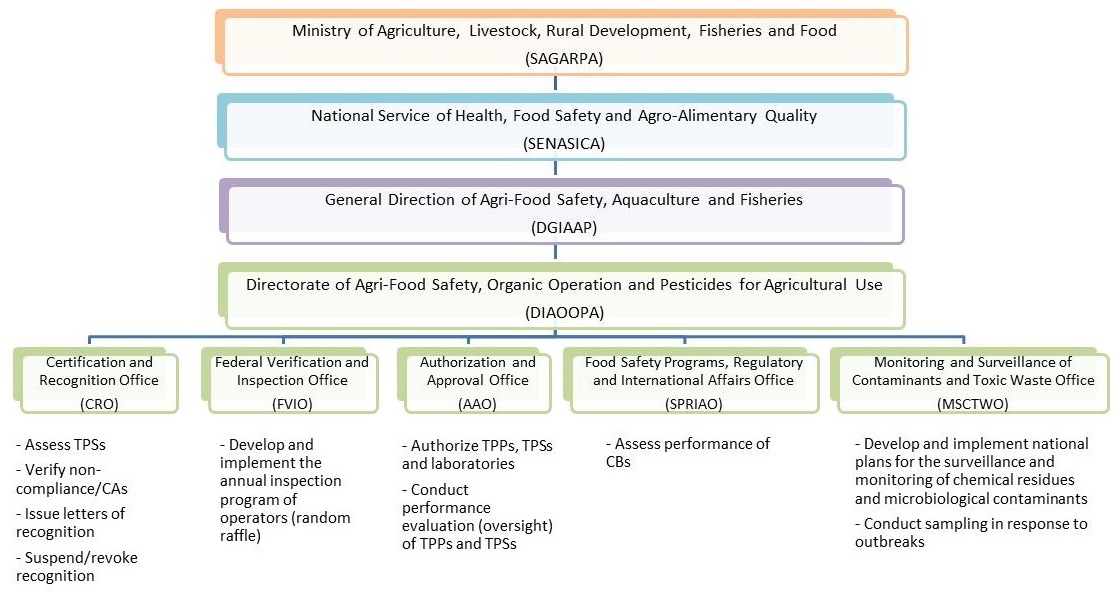
Description of Figure 3: Sample layout (overhead view)
The hierarchy of organizations responsible for the CRRS is as follows (descending from the highest level organization):
- Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA)
- National Service of Health, Food Safety and Agro-Alimentary Quality (SENASICA)
- General Direction of Agri-Food Safety, Aquaculture and Fisheries (DGIAAP)
- Directorate of Agri-Food Safety, Organic Operation and Pesticides for Agricultural Use (DIAOOPA)
-
Certification and Recognition Office (CRO)
Function of the CRO:
- Assess TPSs
- Verify non-compliance/CAs
- Issue letters of recognition
- Suspend/revoke recognition
-
Federal Verification and Inspection Office (FVIO)
Function of the FVIO:
- Develop and implement the annual inspection program of operators (random raffle)
-
Authorization and Approval Office (AAO)
Function of the AAO:
- Authorize TPPs, TPSs and Laboratories
- Conduct performance evaluation (oversight) of TPPs and TPSs
-
Food Safety Programs, Regulatory and International Affairs Office (SPRIAO)
Function of the SPRIAO:
- Assess performance of CBs
-
Monitoring and Surveillance of Contaminants and Toxic Waste Office (MSCTWO)
Function of the MSCTWO:
- Develop and implement national plans for the surveillance and monitoring of chemical residues and microbiological contaminants
- Conduct sampling in response to outbreaks
-
- Directorate of Agri-Food Safety, Organic Operation and Pesticides for Agricultural Use (DIAOOPA)
- General Direction of Agri-Food Safety, Aquaculture and Fisheries (DGIAAP)
- National Service of Health, Food Safety and Agro-Alimentary Quality (SENASICA)
Appendix B. Overview of the CRRS Process (steps and parties involved)
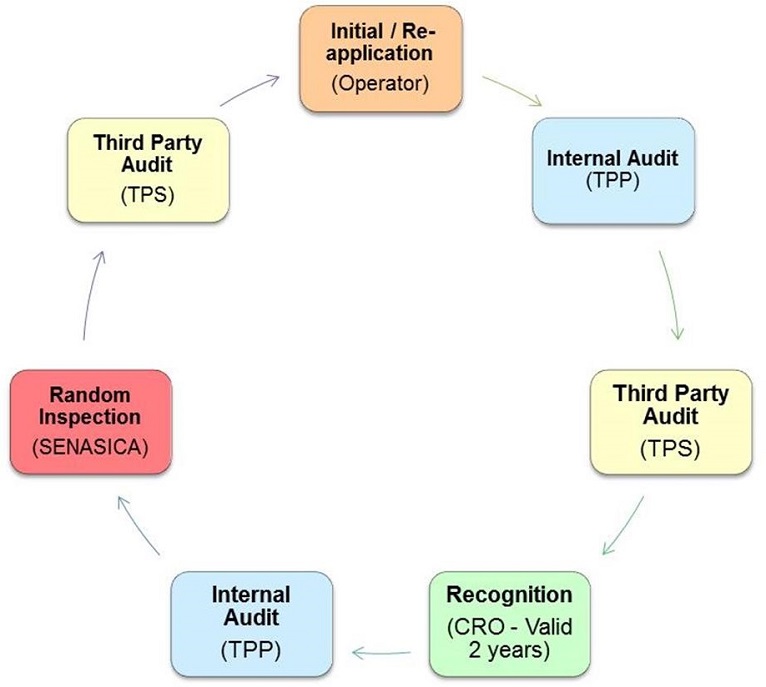
Description of Figure 4: Sample layout (overhead view)
The 8 steps involved in the CRRS process and the person/organization responsible for performing them:
- Step 1: Initial / Re-application (Operator)
- Step 2: Internal audit (TPP)
- Step 3: Third party audit (TPS)
- Step 4: Recognition (CRO - valid 2 years)
- Step 5: Internal audit (TPP)
- Step 6: Random inspection (SENASICA)
- Step 7: Third party audit (TPS)
- Step 8: Return to Step 1.
Appendix C. Summary of SENASICA's action plans/comments to the CFIA's recommendations from the Canadian Food Inspection Agency On-site Assessment Report of Mexico's Food Safety Control System for Fresh Fruit and Vegetables – 2017
| No | CFIA recommendation | SENASICA action plans / comments |
|---|---|---|
| 1. | Revise the 2 year recognition process in order to reduce the administrative burden on all parties and re-allocate funds to areas of higher risk. |
In June, started the review of the recognition process, according the Contamination Risk Reduction System (SRRC) guidelines:
|
| 2. | Review the current processes for the authorization and monitoring of auxiliary parties (TPPs, TPSs, laboratories) to take into consideration the previous history of the individuals/ organizations and consider the potential value of third party certifications, for example, Primus, Global Food Safety Initiative, and the British Retail Consortium. The revised authorization process should focus on results of oversight activities, training received in the past 2 years, and any certifications granted. |
Update the procedure for the authorization of Professionals and Third Party Specialists in SRRC in the production of fresh fruits and vegetables. The updating of the procedure of authorization is considering specific processes for those TPPs and TPSs that are authorized for the first time and those that are renovating their validity. This process will operate by using an informatics system called: Authorization of Auxiliary Parties System, the main objective of this system is to attend the approval application in a very short time but also to have third party specialists and professionals register that allow us to have their documentary records, monitor their activities and historical performance. Activities to be developed Activities for authorization For the renewal of licenses, the manual of procedures will be updated in 2018, the documentary records previously delivered by the TPPs or TPSs, will be considered, in order to avoid having the same documentary information. Also it is expected that the database will also consider the results from the supervisions and performance assessments, activities and training obtained from the last 2 years (during their validity). Activities for monitoring Activities for supervision Include in the Manual of Procedures the supervision of TPPs and TPSs during the certification process in SRRC; consider into account the results of the supervision for the renewal of licenses. Activities of regulatory improvement Develop the "agreement under which are established the general guidelines for presenting the initial notice for operating, and the certification of SRRC in primary unit productions and establishments that made post-harvest activities; and also for the use of the SRRC National Emblem", this document is coordinated with the Directorate of Normalization of SENASICA and could be considered until it is being published in the Federal Official Gazette. |
| 3. | Consider developing a national CB network to identify best practices, share-training resources and other information/experience to ensure consistent national delivery of the program. |
Since 2017, was created at SENASICA the Coordination and Linkage Unit (UCE), which is integrated by officers, divided into regions in every state of Mexico and is in charge of monitoring the working plans of the CB. This unit can be considered as an official communication network under different levels and will be strengthen by using regular teleconferences or video calls. It is also operating a Risk Management Scheme, which is in charge of making annual meetings with people that belongs to CB's and which objective is identify and improve technical assistance, training and share experiences in the development and implementation of the Food Safety Program in each state of the country. It is important to mention that each year are organized work meetings with all the CBs for training, updating and standardizing criteria. Since 2012, SENASICA has celebrated the International Food Safety Forum in which participate more than 1500 people between producers, researchers, representatives or different organizations. Annually, within the framework of this event, we have meetings with CBs to exchange experiences and update the people that work with us by presenting scientific and technical advances that ensure the accomplishment of the Food Safety Program. With respect to the information to ensure the national delivery of the food safety program, since July, 2017 it started the implementation of a single format to monitor the activities of the CBs, this strategy is due to the observations made by our financial regulators. We will also standardize the technical supervision since October 2017. |
| 4. | Consider developing a standardized template for the training and assessment of TPPs and TPSs as well as for recording and tracking employee training to facilitate the identification of training requirements. |
The General Direction Agri-Food Safety, Aquaculture and Fisheries has created the document: "Guidelines for the Offer Agents of Training and Updating Courses for individuals and legal entities", which has the main objective to establish technical criteria and specific requirements for the assessment of academic institutions, organizations and professional colleges, research organizations interested in helping SENASICA for the training and updating purposes of TPPs and TPSs. On June 15, 2017 SENASICA presented the "Guidelines for the Offer Agents of Training and Updating Courses for individuals and legal entities" to universities and academic institutions interested in providing training courses to third party entities. On September 12, 2017, a second meeting with those institutions occurred and training to staff in the certification of SRRC and Good Agricultural Practices was delivered. The Guidelines for the Offer Agents establish the next topics:
|
| 5. | Re-assess the need for the ongoing presence of a TPP in the implementation of the CRRS once an operation has been recognized. |
The presence of the technical staff during and after the company gets its certificate in food safety was analyzed , this activity is coordinated with the Certification and Recognition Office of DIAOOPA (CRO) in the new procedure, which establish:
|
| 6. | Review SENASICA's CRRS oversight process that verifies the requirement for operators to develop and implement a "database on the indicator's behaviour of products, water sources and contact surfaces" to ensure that operators understand what is expected of them and, they have the capacity to conduct trend analysis, etc. |
Since August 2017 we started the process of developing a guideline for the companies on how to generate a database (records) and how to use. This database will be fed by the results of the analysis (physical-chemical, microbiological, pesticide residues, etc) from the units that implement SRRC. On November 2017 we will make a test for the guideline in the production and packinghouse units for detecting opportunity areas and make the necessary adjustments. On December 2017, we will notify to all SRRC stakeholders (farms, packinghouses, TPPs, TPSs and SENASICA's officers in the states) about this Guideline, which will entry into force on January 2018. At the same time, we will include in the Inspection and Verification procedure the specification to verify the database during the visits that will be made in 2018. This will permit to have historical trend of analysis that will be the basis for the improvement and that will allow to the production units and packinghouses to use this for its own performance. In addition, we will train TPPs in SRRC for the identification and elaboration of risk analysis based on the database historical trend. |
| 7. | Review the oversight process for evaluating operator's traceability procedures to ensure that they are well-prepared to respond to real-life issues. For example, mock recalls should include players such as SENSASICA, CBs, etc. and CAs. |
Since July 2017 we started the process of updating the "Guide of the Traceability System of the production of fresh fruits and vegetables in Mexico" in which are included those activities that companies must do to act in case of any sanitary emergency and develop the corresponding procedure as indicated in section 8.6 and 8.7 of the Technical Annex 1. Guidelines for implementing SRRC. In November 2017, we will make a practical exercise in a company to ensure that the elements involved in the procedure could solve properly a sanitary emergency in a real-life event. In December 2017, we will notify to all SRRC stakeholders (farms, packinghouses, TPPs, TPSs and SENASICA's officers in the states) about the update of this Guideline, which will entry into force on January 2018. In 2018, the Federal Inspection and Verification Office of DIAOOPA will make their activities with a special emphasis on this. |
| 8. | Re-consider SENASICA's role in assessing CAs that are generated by TPSs, that is, non-conformities that they have not directly observed. |
Update the methodology establish in "Technical Annex 5. Procedure for Auditing the SRRC in the primary production of fresh fruits and vegetables" and General Guidelines for the Operation and Certification of SRRC in the primary production of Fresh Fruits and Vegetables that use TPSs for the assessment of SRRC. We will establish that in Headquarters we will receive only those assessments reports that are in conditions of issue the certification of the companies. Generate a circular letter through which we will notify all SRRC stakeholders the critical points from the risk analysis, the lapse of time for delivering the assessment reports, but also the documentation that they need to send to SENASICA's Offices, according to the established procedure. Train TPSs so they could have the required tools for determining risks inside production units and harmonize criteria for the assessment of SRRC in the primary production in order to decrease the extraordinary information requirements and extend response timeframes for the companies. |
| 9. | Consider developing new outcome-based tools/checklists to assess/conduct internal audits, third party audits and SENASICA inspections. This will allow the different parties involved to observe the operation from different perspectives, leading to a more comprehensive assessment of their implementation of the CRRS. This should also better prepare operators to respond to food safety issues. |
On June 2017, the first checklist draft for the verification for the assessment of SRRC for TPSs was developed. On October 2017, we will develop the other 2 checklists (Internal Audits and inspections of officers) and on November 2017, we will make a proof with the 3 verification checklists in a farm and packinghouse to assure that the considered points accomplish with the main objective: implement, assess and verify the SRRC. On December 2017, we will notify to all SRRC stakeholders (farms, packinghouses, TPPs, TPSs and SENASICA's officers in the states) about the entry into force of these 3 checklists that we expect that could be during the first trimester or 2018. |
| 10. | Evaluate the needs for additional program resources in order to meet increasing demands as more companies join the program. |
Since April 2017 and due to the increase in the number of companies added to SRRC, DIAOOPA changed its operational structure, creating a single area that attends the proceedings related to SRRC. At present, we are working on the implementation of systematized mechanisms for a better, and greater, information management and analysis. Since August 2017, the electronic register system of the companies that implement SRRC was improved, we are also working on the information migration to an electronic system to know the real progress in the implementation and recognition of SRRC of the companies; we are expecting to issue electronic certificates in the medium and long term. In addition, we are jointly working with food safety private schemes in order to generate equivalence strategies with SRRC so we can consider production units of other schemes as being part of SENASICA. |
| 11. | Review the current prioritization process to allocate resources to activities that are truly critical to the success of the program. |
Financial resources through which Mexican Food Safety Program operates is subject to a set of specific provisions called "Rules for the Operation". Resources are committed to the Collaboration Bodies (CBs) through the Program for Promoting Inversion and Productivity by the program denominated: "Food Safety and Plant Health". Additionally, it exits a Fund for the attention of Sanitary Emergency Events. It is important to note, that for the resources commitment we have done previous analysis, according the surface and the importance level of crops/species, also the contaminants presence or incidence. This process starts with coordination mechanisms that includes the conformation of the information from the contaminants monitoring, noting the next Programs:
|