Specific foods
On this page
- Foods intended solely for infants 6 months of age or older but less than 1 year of age
- Foods for use in manufacturing other foods
- Foods for commercial or industrial enterprise or institution
- Annex 1: Vitamin and mineral declarations
Foods intended solely for infants 6 months of age or older but less than 1 year of age
All formats for infants 6 months of age or older but less than 1 year of age may be used only on foods that are promoted and sold solely for infants 6 months to less than 12 months old. Products that are also marketed to young children or adults are not subject to the special provisions in the regulations that apply to food for infants 6 months of age or older but less than 1 year of age. For example, arrowroot cookies that are typically consumed by both young children and infants 6 months to less than 12 months old are not eligible for the formats described in this section.
This section is not complete on its own. It should be used in conjunction with the Information within the Nutrition Facts table section and with the specific nutrition labelling regulations pertaining to foods intended solely for infants 6 months of age or older but less than 1 year of age found in the Food and Drug Regulations (FDR).
For information on nutrient content claims and health claims on foods for infants 6 months to less than 12 months old, refer to sections Nutrient content claims on foods for children under 4 years of age and Health claims on foods for children under 4 years of age.
When to use a Nutrition Facts table
Requirements
Unless exempted, prepackaged foods intended solely for infants 6 months to less than 12 months old are required to carry a Nutrition Facts table (NFt) [B.01.401(1), FDR].
Prohibitions
The following foods intended solely for infants 6 months to less than 12 months old are specifically prohibited from declaring a Nutrition Facts table [B.01.401(4), B.01.401(5), FDR]:
- infant formula, and
- foods containing infant formula
For more information on the requirements for these products, please refer to the Infant foods and infant formula page.
Exemptions
The same exemptions from displaying a Nutrition Facts table and reasons for losing the exemptions that apply to all other foods also apply to prepackaged foods intended solely for infants 6 months to less than 12 months old.
Manner of declaring
Mandatory information in the Nutrition Facts table
For information on the elements generally included in a Nutrition Facts table, please refer to the Information within the Nutrition Facts table section.
In the case of foods solely for infants 6 months of age or older but less than 1 year of age, the Nutrition Facts table includes [B.01.403, FDR]:
- the title "Nutrition Facts"
- the serving of stated size
- the number of Calories
- the amounts of fat, carbohydrate, fibre, sugars, protein, sodium, potassium, calcium and iron
- the percent daily values (% DV) of potassium, calcium and iron, and
- Note: % DVs for these minerals must be calculated based on the daily value for infants 6 months of age or older but less than 1 year of age, which are set out in column 2 of Part 2 of the Table of Daily Values
- the % DV interpretative statement
There are several major differences between nutrition information requirements for prepackaged food for consumers and prepackaged foods for infants 6 months of age or older but less than 1 year of age [B.01.403, FDR]. These differences are:
- Nutrients listed in the top part of the Nutrition Facts table (fat, fibre, sugars, cholesterol, sodium and the sum of saturated and trans fatty acids) are declared in absolute units only (g, mg). Percent daily values (% DV) are not declared for these nutrients.
- Certain core nutrients may be omitted: saturated fatty acids, trans fatty acids and cholesterol. However, if cholesterol is declared, then saturated and trans fatty acids must also be declared.
- The Nutrition Facts table formats are modified to reflect these differences. For example, there are no dual formats for foods for infants 6 months of age or older but less than 1 year of age.
Serving size
Serving sizes are based on the product as sold and are closely aligned with regulated reference amounts. The Table of Reference Amounts for Food provides reference amounts on which most serving sizes are based, for various categories of food, including "Foods intended solely for children under 4 years of age". It also provides instructions on how to determine and express the serving size to be declared in the Nutrition Facts table.
For more information on reference amounts, refer to the Reference amount section.
Additional information in the Nutrition Facts table
Additional information included in the table following B.01.402 of the FDR may be shown in the Nutrition Facts tables for infants 6 months of age or older but less than 1 year of age. When shown, the additional information shown in the NFt must be presented as illustrated in the figure below [Figures 33.1(E) and(F) or Figure 34.1(B), Nutrition Facts table formats for infants 6 months of age or older but less than 1 year of age section. An example of the standard format in both English and French is also shown below. The additional information must be shown in both English and French, unless otherwise exempted from bilingual labelling requirements.
Standard format – infants 6 months of age or older but less than 1 year of age

Figure 20.1(E)
Left justified at the top of the table is the heading Nutrition Facts in bold. The next line is Per HM open parenthesis MM close parenthesis. There is a rule below Per HM open parenthesis MM close parenthesis that spans the width of the table. The next line is Calories in bold followed by a placeholder, also in bold, for the amount of Calories. There is a thick rule under Calories that ends after the amount placeholder; it does not span the width of the table.
Left justified on the next line is Fat in bold and right justified on the same line is a placeholder for amount of fat followed by a lowercase g. There is a thin rule below the Fat information that spans the width of the table.
The next line is Carbohydrate in bold and right justified on the same line is a placeholder for the amount of carbohydrate followed by a lowercase g. Indented on the next line under Carbohydrate is Fibre and right justified on the same line is a placeholder for the amount of fibre followed by a lowercase g. Indented on the next line under Fibre is Sugars and right justified on the same line is a placeholder for the amount of sugars followed by a lowercase g. There is a thin rule under the Sugars information that spans the width of the table.
The next line is Protein in bold and right justified on the same line is a placeholder for the amount of protein followed by a lowercase g. There is a thin rule under the Protein information that spans the width of the table.
The next line is Sodium in bold and right justified on the same line is a placeholder for the amount of sodium followed by mg in lowercase. There is a thick rule under the Sodium information that spans the width of the table.
Right justified on the next line is the subheading percent symbol Daily Value in bold. Percent Daily Value has an asterisk that refers to a footnote at the bottom of the Nutrition Facts table.
The next line is Potassium followed by a placeholder for the amount of potassium followed by mg in lowercase. Right justified on the same line is a placeholder for percent Daily Value of potassium followed by a percent symbol. There is a thin rule under the Potassium information that spans the width of the table.
The next line is Calcium followed by a placeholder for the amount of calcium followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of calcium followed by a percent symbol. There is a thin rule below the Calcium information that spans the width of the table.
The next line is Iron followed by a placeholder for the amount of iron followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of iron followed by a percent symbol. There is a thick rule under the Iron information that spans the width of the Nutrition Facts table.
The next line is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, with a little in bold. The next line is 15 percent symbol or more is a lot, with a lot in bold. This is the end of the Nutrition Facts table.

Figure 20.1(F)
Left justified at the top of the table is the heading Valeur nutritive in bold. The next line is pour MD open parenthesis MM close parenthesis. There is a rule below pour MD open parenthesis MM close parenthesis that spans the width of the table. The next line is Calories in bold followed by a placeholder, also in bold, for the amount of calories. There is a thick rule under Calories that ends after the amount placeholder; it does not span the width of the table.
Left justified on the next line is Lipides in bold and right justified on the same line is a placeholder for amount of lipides followed by a lowercase g. There is a thin rule below the Lipides information that spans the width of the table.
The next line is Glucides in bold and right justified on the same line is a placeholder for the amount of glucides followed by a lowercase g. Indented on the next line under Glucides is Fibres and right justified on the same line is a placeholder for the amount of fibres followed by a lowercase g. Indented on the next line under Fibres is Sucres and right justified on the same line is a placeholder for the amount of sucres followed by a lowercase g. There is a thin rule under the Sucres information that spans the width of the table.
The next line is Protéines in bold and right justified on the same line is a placeholder for the amount of protéines followed by a lowercase g. There is a thin rule under the Protéines information that spans the width of the table.
The next line is Sodium in bold and right justified on the same line is a placeholder for the amount of sodium followed by mg in lowercase. There is a thick rule under the Sodium information that spans the width of the table.
Right justified on the next line is the subheading percent symbol valeur quotidienne in bold. Percent valeur quotidienne has an asterisk that refers to a footnote at the bottom of the Valeur nutritive table.
The next line is Potassium followed by a placeholder for the amount of potassium followed by mg in lowercase. Right justified on the same line is a placeholder for percent valeur quotidienne of potassium followed by a percent symbol. There is a thin rule under the Potassium information that spans the width of the table.
The next line is Calcium followed by a placeholder for the amount of calcium followed by mg in lowercase. Right justified on the same line is a placeholder for the percent valeur quotidienne of calcium followed by a percent symbol. There is a thin rule below the Calcium information that spans the width of the table.
The next line is Fer followed by a placeholder for the amount of fer followed by mg in lowercase. Right justified on the same line is a placeholder for the percent valeur quotidienne of fer followed by a percent symbol. There is a thick rule under the Fer information that spans the width of the table.
The next line is the percent valeur quotidienne footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, with peu in bold. The next line is 15 percent symbol ou plus c'est beaucoup, with beaucoup in bold. This is the end of the Valeur nutritive table.
Additional information – infants 6 months of age or older but less than 1 year of age
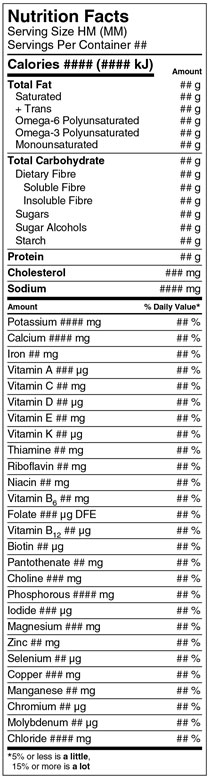
Figure 33.1(E)
Left justified at the top of the table is the heading Nutrition Facts in bold. The next line is Serving Size, followed by HM open parenthesis MM close parenthesis. Stacked below on the next line is Servings Per Container followed by a placeholder for the number of servings. There is a rule below the Servings Per Container that spans the width of the table.
The next line is Calories in bold followed by a placeholder, also in bold, for the amount of Calories per serving of the product. This is followed by open parenthesis number symbol placeholder and letters lowercase k, uppercase J, close parenthesis. Right justified on the same line is the subheading Amount in bold. There is a thick rule under Calories information that ends after the amount placeholder in parentheses; it does not span the width of the table. Outside the table, on the right side and in line with the subheading Amount, there is a curly bracket followed by the word or, followed by Amount Per Serving, in bold.
Left justified on the next line inside the table, is Total Fat in bold and right justified on the same line is a placeholder for amount of total fat followed by a lowercase g. Indented on the next line under Total Fat is Saturated and right justified on the same line is a placeholder for amount of saturated followed by a lowercase g. Indented on the next line under Saturated there is a plus symbol followed by Trans and right justified on the same line is a placeholder for the amount of trans followed by a lowercase g. Indented on the next line is Omega-6 Polyunsaturated and right justified on the same line is a placeholder for the amount of omega-6 polyunsaturated followed by a lowercase g. Indented on the next line under Omega-6 Polyunsaturated is Omega-3 Polyunsaturated and right justified on the same line is a placeholder for the amount of omega-3 polyunsaturated followed by a lowercase g. Indented on the next line under Omega-3 Polyunsaturated is Monounsaturated and right justified on the same line is a placeholder for the amount of monounsaturated followed by a lowercase g. There is a thin rule below the monounsaturated information that spans the width of the table.
The next line is Total Carbohydrate in bold and right justified on the same line is a placeholder for the amount of total carbohydrate followed by a lowercase g. Indented by 6 points on the next line under Total Carbohydrate is Dietary Fibre and right justified on the same line is a placeholder for the amount of dietary fibre followed by a lowercase g. Indented by 12 points on the next line under Dietary Fibre is Soluble Fibre and right justified on the same line is a placeholder for the amount of soluble fibre followed by a lowercase g. Indented by 12 points on the next line under soluble fibre is Insoluble Fibre and right justified on the same line is a placeholder for the amount of insoluble fibre followed by a lowercase g. Indented by 6 pointson the next line under Insoluble Fibre is Sugars and right justified on the same line is a placeholder for the amount of sugars followed by a lowercase g. Indented by 6 points on the next line under Sugars is Sugar Alcohols and right justified on the same line is a placeholder for the amount of sugar alcohols followed by a lowercase g. Indented by 6 points on the next line under Sugar Alcohols is Starch and right justified on the same line is a placeholder for the amount of starch followed by a lowercase g. There is a thin rule under the starch information that spans the width of the table.
The next line is Protein in bold and right justified on the same line is a placeholder for amount of protein followed by a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol in bold and right justified on the same line is a placeholder for the amount of cholesterol followed by mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold and right justified on the same line is a placeholder for the amount followed by mg in lowercase. There is a thick rule under the sodium information that spans the width of the table.
The next line is the subheading Amount in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a rule under the amount and percent daily value subheadings that spans the width of the table. Outside the table, on the left side and in line with the subheading Amount, there is a curly bracket and the word or, followed by Amount Per Serving, in bold.
The next line inside the table, is Potassium followed by a placeholder for the amount of potassium followed by mg in lowercase. Right justified on the same line is a placeholder for percent Daily Value of potassium followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by a placeholder for the amount of calcium followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of calcium followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron followed by a placeholder for the amount of iron followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of iron followed by a percent symbol. There is a thin rule under the iron information that spans the width of the table.
The next line is Vitamin A followed by a placeholder for the amount of vitamin A followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of vitamin A followed by a percent symbol. There is a thin rule under the vitamin A information that spans the width of the table.
The next line is Vitamin C followed by a placeholder for the amount of vitamin C followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of vitamin C followed by a percent symbol. There is a thin rule under the vitamin C information that spans the width of the table.
The next line is Vitamin D followed by a placeholder for the amount of vitamin D followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of vitamin D followed by a percent symbol. There is a thin rule under the vitamin D information that spans the width of the table.
The next line is Vitamin E followed by a placeholder for the amount of vitamin E followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of vitamin E followed by a percent symbol. There is a thin rule under the vitamin E information that spans the width of the table.
The next line is Vitamin K followed by a placeholder for the amount of vitamin K followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of vitamin K followed by a percent symbol. There is a thin rule under the vitamin K information that spans the width of the table.
The next line is Thiamine followed by a placeholder for the amount of thiamine followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of thiamine followed by a percent symbol. There is a thin rule under the thiamine information that spans the width of the table.
The next line is Riboflavin followed by a placeholder for the amount of riboflavin followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of riboflavin followed by a percent symbol. There is a thin rule under the riboflavin information that spans the width of the table.
The next line is Niacin followed by a placeholder for the amount of niacin followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of niacin followed by a percent symbol. There is a thin rule under the niacin information that spans the width of the table.
The next line is Vitamin B6 followed by a placeholder for the amount of vitamin B6 followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of vitamin B6 followed by a percent symbol. There is a thin rule under the vitamin B6 information that spans the width of the table.
The next line is Folate followed by a placeholder for the amount of folate followed by the symbol µ, a lowercase g and the letters DFE. Right justified on the same line is a placeholder for the percent Daily Value of folate followed by a percent symbol. There is a thin rule under the folate information that spans the width of the table.
The next line is Vitamin B12 followed by a placeholder for the amount of vitamin B12 followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of vitamin B12 followed by a percent symbol. There is a thin rule under the vitamin B12 information that spans the width of the table.
The next line is Biotin followed by a placeholder for the amount of biotin followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of biotin followed by a percent symbol. There is a thin rule under the biotin information that spans the width of the table.
The next line is Pantothenate followed by a placeholder for the amount of pantothenate followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of pantothenate followed by a percent symbol. There is a thin rule under the pantothenate information that spans the width of the table.
The next line is Choline followed by a placeholder for the amount of choline followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of choline followed by a percent symbol. There is a thin rule under the choline information that spans the width of the table.
The next line is Phosphorous followed by a placeholder for the amount of phosphorous followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of phosphorous followed by a percent symbol. There is a thin rule under the phosphorous information that spans the width of the table.
The next line is Iodide followed by a placeholder for the amount of iodide followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of iodide followed by a percent symbol. There is a thin rule under the iodide information that spans the width of the table.
The next line is Magnesium followed by a placeholder for the amount of magnesium followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of magnesium followed by a percent symbol. There is a thin rule under the magnesium information that spans the width of the table.
The next line is Zinc followed by a placeholder for the amount of zinc followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of zinc followed by a percent symbol. There is a thin rule under the zinc information that spans the width of the table.
The next line is Selenium followed by a placeholder for the amount of selenium followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of selenium followed by a percent symbol. There is a thin rule under the selenium information that spans the width of the table.
The next line is Copper followed by a placeholder for the amount of copper followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of copper followed by a percent symbol. There is a thin rule under the copper information that spans the width of the table.
The next line is Manganese followed by a placeholder for the amount of manganese followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of manganese followed by a percent symbol. There is a thin rule under the manganese information that spans the width of the table.
The next line is Chromium followed by a placeholder for the amount of chromium followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of chromium followed by a percent symbol. There is a thin rule under the chromium information that spans the width of the table.
The next line is Molybdenum followed by a placeholder for the amount of molybdenum followed by the symbol µ, and a lowercase g. Right justified on the same line is a placeholder for the percent Daily Value of molybdenum followed by a percent symbol. There is a thin rule under the molybdenum information that spans the width of the table.
The next line is Chloride followed by a placeholder for the amount of chloride followed by mg in lowercase. Right justified on the same line is a placeholder for the percent Daily Value of chloride followed by a percent symbol. There is a thick rule under the chloride information that spans the width of the table.
The next line is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, and stacked below is, 15 percent symbol or more is a lot. The terms 'a little' and 'a lot' are in bold. This is the end of the Nutrition Facts table.
Note: this image does not display a format choice.
Nutrition Facts table formats for infants 6 months of age or older but less than 1 year of age
Sections B.01.461 through B.01.465 of the FDR set out the criteria for each of the formats of the NFt for foods intended solely for infants 6 months of age or older but less than 1 year of age. The formats and versions (sizes) are specified in the Considerations for selecting an appropriate Nutrition Facts table section. They are selected in the same manner as for other consumer foods, with some exceptions. For example, there are no dual formats for infants 6 months of age or older but less than 1 year of age.
The acceptable formats of NFt for foods solely for infants 6 months of age or older but less than 1 year of age can be found in the Directory of Nutrition Facts Table Formats, specifically Figures 20.1(E) to 34.1(B).
Steps for choosing a Nutrition Facts table
Step 1: measure the available display surface (ADS) of your package
For information on calculating the ADS of the package, see the Available display surface section.
Step 2: choose an NFt format family
Sections B.01.461 to B.01.464 of the FDR set out "families" of formats applying to infants 6 months of age or older but less than 1 year of age. Each family provides format options for presenting nutrition information in a specific manner.
The table below entitled "Which Nutrition Facts table format family should I use?" is a tool for selecting the appropriate format family for a prepackaged product. Once the appropriate family is determined, the decision tree diagrams provide guidance on the specific template that should be chosen.
| Format | May be used for: | Must be used for: | Example(s) |
|---|---|---|---|
| Standard/horizontal/linear formats – for infants 6 months of age or older but less than 1 year of age [B.01.461, FDR] |
|
|
|
| Simplified formats – for infants 6 months of age or older but less than 1 year of age [B.01.462, FDR] |
|
N/A (always optional) | |
| Aggregate format – different kinds of food – for infants 6 months of age or older but less than 1 year of age [B.01.463, FDR] |
|
|
|
| Aggregate format- different amounts of food– for infants 6 months of age or older but less than 1 year of age [B.01.464, FDR]. |
|
N/A (always optional) |
|
Step 3: choose the appropriate Nutrition facts table using decision trees
Before using the decision trees below, please refer to the Guide to using decision trees in the NFt formats section.
Standard, horizontal, linear formats – infants 6 months of age or older but less than 1 year of age [B.01.461, FDR]
Click on image for larger view
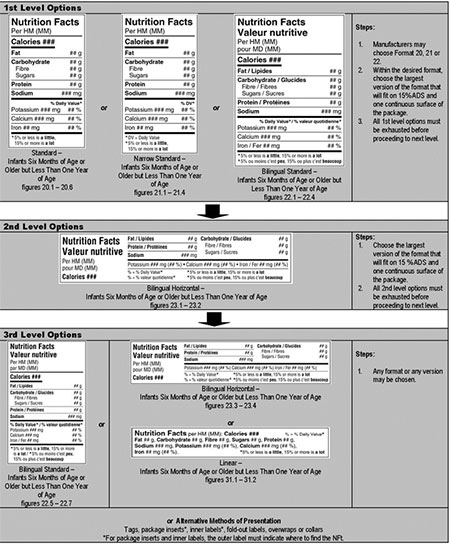
Standard, horizontal, linear formats – infants 6 months of age or older but less than 1 year of age – hierarchy of formats/decision tree
1st Level Options: Steps for choosing between the standard formats
- Manufacturer may choose format 20, 21 or 22. Standard (figure 20.1–20.6), narrow standard (figure 21.1–21.4) or bilingual standard (figure 22.1–22.4).
- Within the desired format, choose the largest version that will fit on 15% ADS and 1 continuous surface of the package.
- All 1st level options must be exhausted before proceeding to next level.
2nd Level Options: Steps for choosing between the horizontal formats
- Choose the largest version of format (figure 23.1–23.2) that will fit on 15% ADS and 1 continuous surface of the package.
- All 2nd level options must be exhausted before proceeding to next level.
3rd Level Options: Steps for choosing between the linear formats
- May choose any format, any version: bilingual standard (figure 22.5-22.7), bilingual horizontal (figure 23.3-23.4), linear (figure 31.1-31.2) or alternative methods of presentations such as tags, package inserts, inner labels, fold-out labels, overwraps or collars.
For package inserts and inner labels, the outer label must indicate where to find the NFt.
Simplified formats – infants 6 months of age or older but less than 1 year of age [B.01.462, FDR]
When used, the simplified formats for foods for infants 6 months of age or older but less than 1 year of age must include the following information [B.01.403(5), FDR]:
- the serving of stated size, the energy value and the amounts of fat, carbohydrate and protein
- the amount of any nutrient which is the subject of a nutritional or health-related claim or representation as described in the Nutrient Content Claims and Health Claims sections
- the amount of any sugar alcohol, vitamin or mineral nutrient added to the food, except fluoride added to prepackaged water or ice
- the amount of fibre, sugars, sodium, potassium, calcium and iron, when these cannot be declared as "0"
- the amount of any vitamin or mineral nutrient declared as a component of 1 of the product's ingredients (except if the ingredient is flour) and
- the statement "Not a significant source of (naming any excluded core nutrients)". Saturated fatty acids, trans fatty acids, and cholesterol are not required to be listed in this statement as their declaration is only triggered when the amount of cholesterol is provided. A shorter version of the above statement is permitted to be used for Nutrition Facts tables in 3rd level options in the decision tree: "Not a significant source of other nutrients"
The % daily value interpretative statement is not required in any figures of the simplified formats – infants 6 months of age or older but less than 1 year of age [B.01.401(8), FDR].
Click on image for larger view

Simplified formats – infants 6 months of age or older but less than 1 year of age – hierarchy of formats/decision tree
1st Level Options: Steps for choosing between the standard formats
- Manufacturer may choose format 24 or 25. Simplified standard (figure 24.1–24.6) or bilingual simplified standard (figure 25.1–25.4).
- Within the desired format, choose the largest version that will fit on 15% ADS and 1 continuous surface of the package.
- All 1st level options must be exhausted before proceeding to next level.
2nd Level Options: Steps for choosing between the horizontal formats
- Choose the largest version of format (figure 26.1–26.2) that will fit on 15% ADS and 1 continuous surface of the package.
- All 2nd level options must be exhausted before proceeding to next level.
3rd Level Options: Steps for choosing between the linear formats
- May choose any format, any version: bilingual simplified standard (figure 25.5-25.6), bilingual simplified horizontal (figure 26.3-26.4), simplified linear (figure 32.1-32.2) or alternative methods of presentations such as tags, package inserts, inner labels, fold-out labels, overwraps or collars.
For package inserts and inner labels, the outer label must indicate where to find the NFt.
Aggregate format – different kinds of foods – infants 6 months of age or older but less than 1 year of age [B.01.463, FDR]
Click on image for larger view
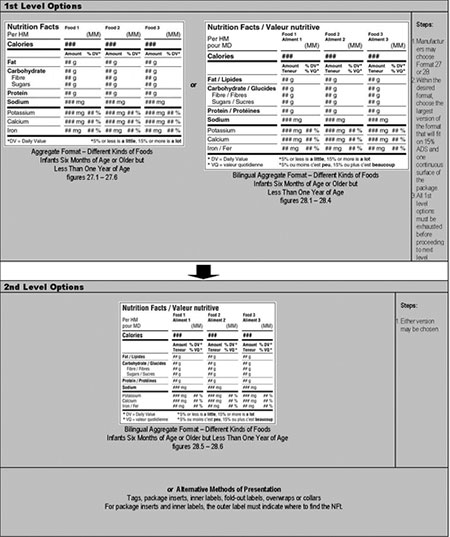
Aggregate format – different kinds of food – infants 6 months of age or older but less than 1 year of age – hierarchy of formats/decision tree
1st Level Options: Steps for choosing between the aggregate formats
- Manufacturer may choose format 27 or 28. Aggregate format (figure 27.1-27.6) or bilingual aggregate format (figure 28.1-28.4).
- Within the desired format, choose the largest version of format that will fit on 15% ADS and 1 continuous surface of the package.
- All 1st level options must be exhausted before proceeding to next level.
2nd Level Options: Steps for choosing a format when 1st level options are exhausted
- May choose any version: bilingual aggregate format (figure 28.5-28.6) or alternative methods of presentation.
Aggregate format – different amounts of food – infants 6 months of age or older but less than 1 year of age [B.01.464, FDR]
Click on image for larger view

Aggregate format – different amounts of food – infants 6 months of age or older but less than 1 year of age – hierarchy of formats/decision tree
1st Level Options: Steps for choosing between the aggregate formats
- Manufacturer may choose format 29 or 30. Aggregate format (figure 29.1-29.6) or bilingual aggregate format (figure 30.1-30.4).
- Within the desired format, choose the largest version of format that will fit on 15% ADS and 1 continuous surface of the package.
- All 1st level options must be exhausted before proceeding to next level.
2nd Level Options: Steps for choosing a format when 1st level options are exhausted
- May choose either version of bilingual aggregate format (figure 30.5-30.6).
- If no version fits, return to hierarchy of format for standard/horizontal/linear formats.
Small packages – infants 6 months of age or older but less than 1 year of age
Foods intended solely for infants 6 months of age or older but less than 1 year of age in small packages with an ADS < 100 cm2 are exempt from carrying a Nutrition Facts table as long as the information is provided as outlined in the small packages < 100 cm2 section.
Front-of-package (FOP) nutrition symbol
Foods intended solely for infants 6 months of age or older but less than 1 year of age are prohibited from carrying an FOP nutrition symbol on their label [B.01.350(15)(a), FDR].
For more information, refer to Foods prohibited from displaying the front-of-package (FOP) nutrition symbol.
Foods for use in manufacturing other foods
This section deals with the requirements of prepackaged foods for use in manufacturing other foods and it identifies the major differences in the presentation of nutrition information for this class of foods as compared to prepackaged foods for the consumer.
Foods for use in manufacturing other foods are prepackaged products that are intended for use as an ingredient [B.01.404 (1), FDR]:
- in the manufacture of other prepackaged products intended for sale to a consumer at the retail level, or
- in the preparation of foods by a commercial or industrial enterprise or institution
This category of foods:
- requires cooking before consumption, or
Note: while these may not strictly be ingredients in the preparation of other foods, their nutrition information may change after cooking or they may be combined with other ingredients (for example, oil) during the cooking process - is to be combined with another ingredient to prepare a food
Examples of foods in this category include: unbaked lasagna, raw seasoned fillets, dry noodles, frozen fries, unbaked pies, canned pie filling, instant potatoes, dried soup mix, corn starch and sugar.
Not all bulk containers of food are considered to be "solely" for use in the manufacturing of other foods. This is determined by the intended use of the ingredients. For example, if the contents are intended to be repacked from bulk at the retail premises or sold directly to consumers from bulk bins, these products require a standardized Nutrition Facts table format.
Nutrition labelling requirements
The nutrition labelling requirements for foods for use in manufacturing other foods are similar to those of prepackaged foods sold to the consumer, with some differences. The following rules apply:
Required nutrients
- nutrition information for energy and the same core nutrients as required for prepackaged foods for the consumer in B.01.401 of the FDR must be included [B.01.404(3)(a), FDR]
- additional information for the same nutrients as required for prepackaged foods for the consumer in section B.01.402 of the FDR must also be provided when triggered [B.01.404(3)(a), FDR]
- additional information permitted in section B.01.402 of the FDR may also be included [B.01.404(3)(b), FDR]
Format
- the information does not have to appear in a Nutrition Facts table format as prescribed by B.01.401(1) of the FDR. Information is not required to be in a box, nor must it obey the formatting requirements [B.01.401(7)(b), FDR]
- the information may simply list the nutrients and their values
No exemption from providing nutrition information
Nutrition labelling exemptions found in B.01.401 of the FDR do not apply to these products [B.01.404(2), FDR]. For example, while prepackaged fresh apples sold in a retail store are generally exempt from carrying a Nutrition Facts table [B.01.401(2)(c)(i), FDR], prepackaged apples intended solely as:
- an ingredient for use in the manufacture of other prepackaged consumer foods (for example, apple sauce), or
- an ingredient (for example, sliced apples) for use in the preparation of food (for example, an apple pie) by a commercial or industrial enterprise or institution
must be accompanied by written nutrition information upon delivery [B.01.404(2), FDR].
Nutrient declarations
Nutrients must be declared:
- per gram (g) or 100 grams (100 g) if the net quantity is declared by weight or count [B.01.404(3)(c)(i)(A) and (ii)(A), FDR], and
- per millilitre (ml) or 100 millilitres (100 ml) if the net quantity of the food is declared by volume [B.01.404(3)(c)(i)(B) and (ii)(B), FDR]
Units of measure
- vitamins must be declared in the units set out in subsection D.01.003(1) of the FDR (that is, mg, µg, µg DFE) [B.01.404(3)(c)(i), FDR]
- minerals must be declared in milligrams for sodium, potassium, calcium, phosphorus, magnesium, iron, zinc, chloride, copper and manganese and in micrograms for iodide, chromium, selenium and molybdenum [B.01.404(3)(c)(i), FDR]
- the information for the other nutrients and energy must be declared in absolute units as set out in column 3 to the tables to B.01.401 and B.01.402 of the FDR (for example, Calories, g, mg)
- the declaration of % daily value and information on "serving of stated size" may be omitted [B.01.404(3)(c)(iii), FDR]
The table in Annex 1: Vitamin and mineral declarations summarizes the units of measure to be used when providing nutrition information for vitamins and minerals for foods for use in manufacturing other foods.
Precision of nutrient declarations and rounding
- all information must be stated with a degree of precision (that is to say, same number of significant figures) corresponding to the accuracy of the analytical methodology used to produce the nutrition information [B.01.404(3)(c)(iv), FDR]
- since the nutrient information provided to the manufacturer may be used to create a Nutrition Facts table for another food, it must not be rounded
- it is also acceptable to declare < (the limit of detection) as opposed to declaring 0, where the declaration is to be to the "degree of precision that corresponds to the accuracy of the analytical methodology used". For example, < 10 mg is acceptable if the nutrient is not detected but the limit of detection is 10 mg
Front-of-package (FOP) nutrition symbol
The following are fully exempt from the FOP labelling requirements:
- products intended only to be used as ingredients in the manufacture of other prepackaged products to be sold to consumers at retail, or
- products intended only to be used as ingredients in the preparation of food by a commercial or industrial enterprise or institution
These products are always exempt from the need to assess the saturated fat, sugars and sodium content against the appropriate threshold. Therefore, they are never required to carry the symbol, even if the nutrient content meets or exceeds the threshold [B.01.350(5)(e), FDR].
For more information, refer to Which foods have a full exemption? in the Front-of-package nutrition symbol labelling guide for industry.
Voluntary claims and statements
Nutrient content claims
The provisions set out in the Food and Drug Regulations for making a nutrient content claim is intended to apply specifically to products sold to consumers at the retail level and where a Nutrition Facts table is required as per B.01.401 of the FDR. However, there is no prohibition from making a nutrient content claim, such as "trans fat free", on foods intended for further manufacture. These types of claims may be made at the manufacturer level provided that the amount of the nutrient subject to the claim, per serving of stated size, is given and the product meets the criteria set out for the claim. Refer to Nutrient content claims for specific requirements regarding these types of claims.
Note: the final product must be assessed independently for compliance with any given claim. Variation in the amount of the ingredient used or the effect of other ingredients used may have an impact on the final product meeting the criteria for the claim.
Documentation for purchaser
In the case of foods that are shipped to a purchaser on a continual basis, with no change to the formulation, documentation may be provided to the purchaser on the basis of the first shipment, without having to provide the information on an ongoing basis provided the purchaser agrees in writing to this arrangement (see Accompanying documentation for nutrition labelling, letter to industry).
Any change to the nutrition information as a result of formulation changes or other influences would have to accompany the modified product with its first delivery after the change has occurred. It is recommended that a reference system be set up to ensure a match between the nutrition information and the incoming material for document control purposes. The purchaser should retain relevant hard copies of the information on file for ingredients that have been used in existing production lots still on the market.
Foods for commercial or industrial enterprise or institution
This section deals with the requirements of ready-to-serve multiple-serving prepackaged products served in a commercial or industrial enterprise or institution. It identifies the major differences in the presentation of nutrition information for this class of foods as compared to prepackaged foods for the consumer.
This category of foods applies to prepackaged products that are [B.01.405 (1), FDR]:
- in multiple servings in a package
- ready to serve (may be shipped frozen or thawed but must be precooked, if required)
- able to be served without the addition of other ingredients (but may be served in conjunction with other foods), and
- intended solely to be served in a commercial or industrial enterprise or institution (for example, a restaurant, cafeteria or hospital)
Examples of this category of foods include: cooked lasagna, gravy, cooked seasoned filets, fresh pasta, pasta sauce, beverages, cherry pie, bagels, cereals, jam, lunch meats, condiments and dressings.
Nutrition labelling requirements
There are many similarities between this category and foods for use in manufacturing other foods. For example, they have the same nutrition labelling requirements with some flexibility in the manner by which nutritional information is provided for a shipment of food (documentation for purchaser) [B.01.401(7)(b), B.01.405(2), B.01.405(3), FDR].
Note: the Nutrient declarations section of foods for use in manufacturing other foods does not apply to ready-to-serve multiple-serving prepackaged foods intended solely to be served in a commercial or industrial enterprise or an institution. Instead, the manner in which the nutrients are declared is the same as for consumer prepackaged products:
- nutrients must include serving size declaration (household measure and metric measure)
- units of measure, rounding and daily values for consumer foods are used
Front-of-package (FOP) nutrition symbol
Ready-to-serve multiple-serving prepackaged products, intended only to be served in a commercial or industrial enterprise or an institution, have a full exemption from the FOP nutrition labelling requirement. These products are always exempt from the need to assess the saturated fat, sugars and sodium content against the appropriate threshold. Therefore, they are never required to carry the symbol, even if the nutrient content meets or exceeds the threshold [B.01.350(5)(d), FDR].
For more information, refer to Which foods have a full exemption? in the Front-of-package nutrition symbol labelling guide for industry.
Annex 1: Vitamin and mineral declarations
| Vitamin | Unit |
|---|---|
| Biotin | µg |
| Choline | mg |
| Folacin or folate | µg DFE |
| Niacin | mg |
| Pantothenic acid or pantothenate | mg |
| Riboflavin or vitamin B2 | mg |
| Thiamin, thiamine, or vitamin B1 | mg |
| Vitamin A | µg |
| Vitamin B6 | mg |
| Vitamin B12 | µg |
| Vitamin C | mg |
| Vitamin D | µg |
| Vitamin E | mg |
| Vitamin K | µg |
| Mineral | Unit |
|---|---|
| Calcium | mg |
| Chloride | mg |
| Chromium | µg |
| Copper | mg |
| Iodide | µg |
| Iron | mg |
| Magnesium | mg |
| Manganese | mg |
| Molybdenum | µg |
| Phosphorus | mg |
| Potassium | mg |
| Selenium | µg |
| Sodium | mg |
| Zinc | mg |
mg = milligrams
µg = micrograms
DFE = dietary folate equivalents
- Date modified: