On this page
- Summary
-
Part 1 - Nutrition labelling compliance test
- Purpose and scope
- Guiding principles
- Statistical basis
- Definitions
- Nutrient definitions
- Methods of analysis
- Application of rounding rules
- Classes of nutrients
- Sampling
- Acceptance criteria for lot compliance
- Tolerances (Criterion 2)
- Use of data bases
- Implementation
- Table - Sampling plan and tolerances
- Examples
- Part 2 - Analysis and feedback
- Appendix 1 - International context
- Appendix 2 - Statistical framework
- Appendix 3 - Rounding of nutrition facts table information
- Appendix 4 - Laboratory issues
Summary
The Food and Drug Regulations set out requirements for nutrition labelling and for the making of nutrient content claims and health claims. These regulatory requirements aim to provide Canadians with information to prevent injury to their health by helping them make appropriate food choices.
The Canadian Food Inspection Agency (CFIA) is responsible for enforcing the food requirements of the Food and Drugs Act and Regulations. While industry is responsible for complying with the regulations, Health Canada and the CFIA are committed to facilitating their implementation in a manner that will retain the confidence of consumers and health professionals in the reliability of the nutrition information.
The challenges for industry in generating product-specific nutrient data for nutrition labelling are recognized. Industry is responsible for ensuring the accuracy of label values and may choose the risk management strategy best suited to the food(s) to be labelled.
The purpose of the CFIA Compliance Test is to provide a transparent, science-based system for verifying the accuracy of nutrient values on labels and in advertising via laboratory analysis as part of assessing compliance with the Food and Drug Regulations.
The Compliance Test constitutes the CFIA methodology for assessing the accuracy of nutrition labelling and claims. It is based on the laboratory analysis of the nutrient content of three composite samples of four consumer units each, randomly selected from a lot and the results of analysis subjected to three acceptance criteria. The principal acceptance criterion would require accuracy within 20% of declared value for the average of three composite samples for naturally occurring nutrients in the Nutrition Facts table, i.e., the analyzed nutrient content would have to be at least 80% of declared value for protein, carbohydrate, fibre, vitamins and minerals and not more than 120% of declared value for Calories, fat, saturated fat, trans fat, cholesterol, sugars and sodium. For added vitamins, mineral nutrients and amino acids in claims or in the Nutrition Facts table, the amount found in the sample must be at least equal to the label value. In addition, adjustments are made for rounding in accordance with rounding rules in the Food and Drug Regulations. Acceptance criteria for overall variability of nutrient levels also apply.
This test does not apply to a human milk substitute, a food represented as containing a human milk substitute, a formulated liquid diet, a meal replacement, a nutritional supplement, a food represented for use in a very low energy diet, or minimum or maximum requirement for added nutrients, which are subject to their own regulations.
Part 1 - Nutrition labelling compliance test
Purpose and scope
The purpose of the Nutrition Labelling Compliance Test (Compliance Test) is to provide a transparent, science-based system for assessing the accuracy of nutrient information for food in Canada.
The Compliance Test outlines the Canadian Food Inspection Agency's (CFIA) procedure for assessing the accuracy of nutrient values on food labels and in advertising via laboratory analysis. The test applies to a nutrient, including an added vitamin or mineral nutrient, that is declared in the Nutrition Facts table or is the subject of a nutrient content claim or a disease risk reduction claim. The Compliance Test also assesses whether a food bearing a nutrient content claim or health claim meets the nutrient content criteria for the claim set out in the Food and Drug Regulations.
The Compliance Test does not apply to a human milk substitute, a food represented as containing a human milk substitute, a formulated liquid diet, a meal replacement, a nutritional supplement, a food represented for use in a very low energy diet, or a minimum or maximum requirement for an added vitamin, mineral nutrient or amino acid.
Guiding principles
To achieve label information that is accurate and in compliance with the Food and Drugs Act and Regulations, the following general principles apply:
- Industry is responsible for ensuring that nutrition labelling and claims are compliant with the Food and Drug Regulations and that label values accurately reflect the nutrient content of the product.
- A suitable compliance test for the accuracy of declared nutrient values must take into consideration the inherent variability of nutrients in foods and the variability of the laboratory method using appropriate statistical analysis.
- The CFIA compliance action will take into consideration not only laboratory results, but also the health risk to the public, economic loss to consumers, past compliance history of the product and the company's quality control over the manufacturing and labelling processes.
Statistical basis
The CFIA considers that the measurement of nutrient content for compliance purposes should be based on a sound statistical framework, such that the food industry would have a high probability of a correct label value passing the Compliance Test, while the consumer would have an equally high probability that the label value accurately reflects the nutrient content of the food. To this end, the Compliance Test is comprised of three acceptance criteria applied to the results of laboratory analysis of samples obtained by using a randomized sampling plan. The statistical analysis takes into account nutrient variability in foods as well as method variability. The producer's risk (the probability that a lot with acceptable label values is erroneously rejected) and the consumer's risk (the probability that a lot with unacceptable label values is erroneously accepted) are calculated using several values for each component of variability (see statistical framework in Appendix 2).
Definitions
Lot: a collection of identically labelledFootnote 1 products produced under conditions as nearly uniform as possibleFootnote 2 and available for inspection at one time.
Consumer unit: the individual container (a primary container) or a portion of the contents of the primary container.
Sample: a collection of consumer units drawn from a lot that is representative of the lot for inspection purposes.
Composite sub-sample: a subset of consumer units from the sample that are combined and mixed to homogeneity.
Nutrient definitions
Calorie calculations and nutrient definitions are found in the Food and Drug Regulations and in Elements within the Nutrition Facts Table.
Methods of analysis
Nutrients are analyzed by appropriate methods found in the most recent edition of Official Methods of Analysis of AOAC International or other collaboratively studied methods wherever possible (Refer to Laboratory Issues in Appendix 4).
Application of rounding rules
Under the rounding rules for nutrition labelling, as set out in the tables to sections B.01.401 and B.01.402 of the Food and Drug Regulations, and summarized in rounding rules, the declared label value is a single rounded value that represents an accepted range of values. The tolerances described below are added to the minimum or maximum pre-rounded value, to obtain the compliance limit (see rounding rules and calculation of compliance limit in Appendix 3).
Classes of nutrients
Class I: a vitamin or mineral nutrient that is added
Class II: a nutrient, other than an added vitamin or mineral nutrient that is in the Nutrition Facts table or that is subject to regulations for nutrient content claims or diet-related health claims.
Sampling
At least twelve individual consumer units are taken randomly from a lot and then combined to make three composite sub-samples of a minimum of four consumer units each. The three composite samples are analyzed and the mean value of the three composite sub-samples shall be the estimate of the lot nutrient content.
Acceptance criteria for lot compliance
The lot will be considered compliant if the following criteria are met (see rationale in Appendix 2):
Criterion 1: Each of the composite sub-samples of four consumer units is within 50% of the declared value expressed as a minimum or maximum value allowed by the appropriate rounding rules, and within 50% of the regulatory requirement, if any; that is, at least half as large or at most 1.5 times as large as the declared value adjusted for rounding and as the regulatory requirement (when a minimum or maximum content is specified respectively).
Criterion 2: The mean ( ) of the three composite sub-samples of four consumer units must fall within the minimum or maximum allowable (compliance) limit, which is the declared nutrient value or regulatory requirement, expressed as a minimum or maximum value allowed by the appropriate rounding rules, if any, with a further tolerance, if any, for that class and nutrient, applied to it (see examples in Appendix 3).
) of the three composite sub-samples of four consumer units must fall within the minimum or maximum allowable (compliance) limit, which is the declared nutrient value or regulatory requirement, expressed as a minimum or maximum value allowed by the appropriate rounding rules, if any, with a further tolerance, if any, for that class and nutrient, applied to it (see examples in Appendix 3).
Criterion 3 (Class I nutrients only): Where a vitamin or mineral nutrient is added to a food, the standard deviation (s) of the distribution of the nutrient content of the three composites of four consumer units, is compared to the mean value ( ) by ensuring that a 10% difference from the mean value lies within a 99% confidence interval for the standard deviation of the lot nutrient content. The 99% lower confidence limit evaluated as (s × 0.4344/
) by ensuring that a 10% difference from the mean value lies within a 99% confidence interval for the standard deviation of the lot nutrient content. The 99% lower confidence limit evaluated as (s × 0.4344/ ) should be less than 0.1 or 10%.
) should be less than 0.1 or 10%.
Tolerances (Criterion 2)
Class I nutrients (no tolerance):
For added vitamins and mineral nutrients, listed in the table to section D.03.002, Food and Drug Regulations, and summarized in Foods to which vitamins, mineral nutrients and amino acids may or must be added, the declared nutrient value has no toleranceFootnote 3, i.e., the mean nutrient content of the three composite sub-samples is not less than the declared value, adjusted for rounding as in acceptance criterion 2.
Class II nutrients: (20% tolerance)
For vitamins and mineral nutrients other than Class I nutrients, protein, polyunsaturated fatty acids, omega-3 fatty acids, omega-6 fatty acids, mono-unsaturated fatty acids, carbohydrate, fibre, soluble fibre, insoluble fibre and potassium, that are declared in the Nutrition Facts table or that are the subject of a nutrient content claim or of a disease risk reduction claim:
- the regulated limit for the claim has a 20% tolerance, i.e. the mean nutrient content of the three composite sub-samples is not less than 80% of the minimum nutrient level required by the Food and Drug Regulations; and
- the declared label value has a tolerance of 20%, i.e., the mean nutrient content of the three composite sub-samples is not less than 80% of the declared value, adjusted for rounding.
For Calories, fat, saturated fat, trans fat, cholesterol, sodium, sugars and sugar alcohols, that are declared in the Nutrition Facts table or that are the subject of a nutrient content claim or of a disease risk reduction claim:
- the regulated limit for the claim has a 20% tolerance; i.e., the mean nutrient content of the three composite sub-samples is not greater than 120% the maximum nutrient level permitted in theFood and Drug Regulations; and
- the declared nutrient value has a tolerance of 20%, i.e., the mean nutrient content of the three composite sub-samples is not more than 120% of the declared value, adjusted for rounding.
Exceptions to class II
Stricter tolerances may be applied, as assessed on a case by case basis, as follows:
- Where the difference between the declared nutrient value and the compliance limit for the nutrient content of the sample may result in a label value that would be a potential risk to health, based on a health risk assessment established by Health Canada.
- Where a comparative claim is found not to be statistically valid, i.e. where providing tolerances allows the food bearing the claim to be indistinguishable from the reference food.
Overages and deficiencies - Class I and II nutrients
Where a minimum limit applies, the nutrient content of the sample may exceed the declared value by an amount that is consistent with good manufacturing practices, and provided that such an overage does not present a risk to health and is not misleading.
Where a maximum limit applies, the nutrient content of the sample may be below the declared value by an amount that is consistent with good manufacturing practices and provided that such a deficiency does not present a risk to health and is not misleading.
Use of data bases
Industry is responsible for complying with all the requirements for nutrient composition and for the accuracy of the information provided on labels. Companies may choose the risk management strategy for developing accurate nutrient data best suited to the foods to be labelled. The use of nutrient data bases is one tool within this context.
To assist manufacturers of multi-ingredient foods, the Food and Drug Regulations require that food ingredients intended solely for use in the manufacture of other prepackaged foods must provide relevant nutrition information about their product [B.01.404, FDR].
Verification of label values by the CFIA will focus on industry system controls, including record keeping, raw material control and specifications, company lab analysis, documentation of data sources, audit verification, management of ingredient data (including updates, ingredient changes, substitutions and processing effects). The CFIA will not evaluate nutrition labelling data bases, as such. The definitive determination of compliance of label values by the CFIA will be based on laboratory analysis, as outlined in the Compliance Test. A tolerance of 20% is allowed in recognition of the variability inherent in nutrient concentrations and to encourage manufacturers to label the food with the true lot average. Where variation is very high, a conservative label value would avoid exceeding the compliance limit. If any product is found to be out of compliance, the CFIA intends to work with the manufacturer to understand and correct the problem.
Implementation
The CFIA Compliance Test applies to a nutrient, including an added vitamin or mineral nutrient, that is declared in the Nutrition Facts table or is the subject of a nutrient content claim or a disease risk reduction claim.
The approach to assessing compliance of nutrient values for raw single ingredient foods for which nutrition labelling is voluntary will be reviewed when adequate data becomes available for these products.
Table - Sampling plan and tolerances
| Class | Description | Nutrients | Acceptance criterion 1, Table note a, Table note b sub-sample | Acceptance criterion 2 Tolerances Table note a, Table note b | Acceptance criterion 3, 99% confidence interval Table note d |
|---|---|---|---|---|---|
| Class I (min) Table note c |
added nutrients (e.g. added vitamin C) |
added vitamins, mineral nutrients, amino acids |
each sub-sample ≥ 50% declared nutrient value |
≥ declared nutrient value |
[(s × 0.4344) ÷ x̅] ≤ 0.1 |
|
Class II (min) Table note c |
a naturally occurring nutrient that is declared in the Nutrition Facts table and/or for which a health or nutrient content claim is made. |
protein, polyunsaturated fatty acids, omega 3 fatty acids, omega 6 fatty acids, mono-unsaturated fatty acids, carbohydrate, starch, fibre, soluble fibre, insoluble fibre, vitamins and minerals (e.g. potassium) |
each sub-sample ≥ 50% declared nutrient value |
≥ 80% declared nutrient value |
does not apply |
|
Class II (max) Table note c |
a naturally occurring nutrient declared in the Nutrition Facts table and/or for which a health or nutrient content claim is made. |
Calories, fat, saturated fat, trans fat, cholesterol, sodium, sugars, sugar alcohols |
≤ 150% declared nutrient value |
≤ 120% declared nutrient value |
does not apply |
Examples
The following examples show how the three criteria of the Compliance Test would be used to assess the accuracy of declared nutrient values from laboratory analysis data. For each scenario, the following information is shown: the amount of nutrient declared in the Nutrition Facts table and/or required for the nutrient content claim; the laboratory analysis of three sub-samples, mean value and standard deviation (s); and the assessment based on one or all of the criteria and justification.
1. Vegetable oil - Fat and fatty acids
Consider the example of vegetable oil to assess a claim about a class II nutrient: "trans fat free"
- Nutrition Facts table declarations per 10 mL: fat = 9 g, saturated fat = 0.5 g, trans fat = 0 g; claim "trans fat free".
This claim has conditions related to fat and saturated fat, so these nutrients must be assessed as well. According to the Table - Sampling plan and tolerances, maximum pre-rounded values must be used for the assessment of fat, saturated fat and trans fat.
Fat
- Laboratory results: sub-samples = 8.9 g, 9.1 g, 9.0 g; mean = 9.0 g; standard deviation (s) = 0.1 g
- Compliance limit criterion 1 for declared value:
(maximum pre-round + 50% of declared value) = [9.4 g + (0.5 × 9 g)] = 13.9 g - Compliance limit criterion 2 for declared value:
(maximum pre-round + 20% of declared value for class II) = [9.4 g + (0.2 × 9 g)] = 11.2 g - Assessment:
criterion 1: compliant: each sub-sample < 13.9 g
criterion 2: compliant: 9.0 g (mean) = 9 g (declared) (which is < 11.2 g)
Saturated fat
- Laboratory results: sub-samples = 0.62 g, 0.65 g, 0.62 g; mean = 0.63 g; s = 0.017 g
- Compliance limit criterion 1 for declared value:
(maximum pre-round + 50% of declared value) = [0.74 g + (0.5 × 0.5 g)] = 0.99 g - Compliance limit criterion 2 for declared value:
(maximum pre-round + 20% of declared value for class II) = [0.74 g + (0.2 ×0.5 g)] = 0.84 g
(see Appendix 3, Table 3) - Assessment:
criterion 1: compliant: each sub-sample < 0.99 g
criterion 2: compliant: 0.63 g (mean) < 0.84 g
Trans fat
- Laboratory results: sub-samples = 0.28 g, 0.28 g, 0.3 g; mean = 0.29 g; s = 0.01 g
- Compliance limit criterion 1:
(maximum pre-round + 50% of 0.2 g) = [0.199 g + (0.5 × 0.2 g)] = 0.3 g - Compliance limit criterion 2:
(maximum pre-round + 20% of 0.2 g for class II) = [0.199 g + (0.2 × 0.2 g)] = 0.24 g - Assessment:
criterion 1: compliant: each sub-sample ≤ 0.3 g
criterion 2: non-compliant, for Nutrition Facts table declaration and "trans fat free" claim: 0.29 g (mean) > 0.24 g
2. Lean ground beef - Iron
Consider the example of lean ground beef to assess the declaration in the NFt about a class II nutrient: Iron
- NFt declaration per 100 g serving: Iron = 1.75 mg = 10 % DV
According to the Table - Sampling plan and tolerances, minimum pre-rounded values must be used for the assessment of iron.
- Laboratory results: sub-samples = 1.4 mg, 1.5 mg, 1.6 mg; mean = 1.5 mg; s = 0.1 mg
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value) = [1.625 mg − (0.5 x 1.75 mg)] = 0.75 mg - Compliance limit criterion 2 for declared value:
(minimum pre-round − 20% of declared value for class II) = [1.625 mg − (0.2 ×1.75 mg)] = 1.275 mg (see Appendix 3, Table 3) - Assessment:
criterion 1: compliant: each sub-sample > 0.75 mg
criterion 2: compliant: 1.5 mg (mean) > 1.275 mg
Since the declared value of 1.75 mg iron is compliant, the % DV declared must now be assessed.
- Assessment of declared % daily value (DV): For iron, the % DV is based on the rounded amount, by weight, of the nutrient, which is 1.75 mg declared.
- DV for iron as per Column 4 of Part 2 of the Table of daily values = 18 mg
- Rounded amount = 1.75 mg
(1.75 mg ÷ 18 mg) × 100 = 9.72 % is rounded to 10 % DV as per rounding rules (see Appendix 3, Table 1), which corresponds to the % DV declared, therefore compliant.
3. Granola cereal - Fibre
Consider the example of granola cereal to assess the declaration in the NFt about a class II nutrient: Fibre
- NFt declaration per 55 g serving: Fibre = 4 g = 13 % DV
According to the Table - Sampling plan and tolerances, minimum pre-rounded values must be used for the assessment of fibre.
- Laboratory results: sub-samples = 2.4 g, 3.3 g, 3.5 g; mean = 3.1 g; s = 0.6 g
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value ) = [3.5 g − (0.5 × 4 g)] = 1.5 g - Compliance limit criterion 2 for declared value:
(minimum pre-round − 20% of declared value for class II) = [3.5 g − (0.2 × 4 g)] = 2.7 g (see Appendix 3, Table 3) - Assessment:
criterion 1: compliant: each sub-sample > 1.5 g
criterion 2: compliant: 3.1 mg (mean) > 2.7 g
Since the declared value of 4 g fibre is compliant, the % DV declared must now be assessed.
- Assessment of declared % daily value (DV): For macronutrients such as fibre, the manufacturer may calculate the % DV based on rounded or unrounded amounts, by weight, of each nutrient. Compliance of the % DV must therefore be assessed in terms of both the rounded amount of 4 g fibre declared, and the minimum pre-round value of 3.5 g fibre.
- DV for fibre as per Column 3 of Part 1 of the Table of daily values = 28 g
- Calculation based on rounded amount = 4 g
(4 g ÷ 28 g) × 100 = 14.28 % is rounded to 14 % DV as per rounding rules (see Appendix 3, Table 1). Before assessing compliance, we must also calculate % DV based on the minimum pre-round value. - Calculation based on minimum pre-round = 3.5 g
(3.5 g ÷ 28 g) × 100 = 12.5 % is rounded to 13 % DV as per rounding rules, which corresponds to the % DV declared. This is deemed compliant (since for macronutrients, the % DV may either be calculated based on rounded or unrounded amounts, by weight).
4. Pasta - Added iron
Consider the example of pasta to assess the declaration in the NFt about a class I nutrient: Added iron
- NFt declaration per 85 g serving: Iron = 2.5 mg Footnote * = 14 % DV
- Laboratory results: sub-samples = 2.42 mg, 2.51 mg, 2.47 mg; mean = 2.467 mg; s = 0.045 mg
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value) = [2.375 mg − (0.5 × 2.5 mg)] = 1.125 mg - Compliance limit criterion 2: Class I = pre-round minimum limit (no tolerance) = 2.375 mg (see Appendix 3, Table 2)
- Assessment:
criterion 1: compliant: each sub-sample > 1.125 mg
criterion 2: compliant: 2.467 mg (mean) > 2.375 mg
criterion 3:compliant: [(0.4344 × s) ÷ mean] = [(0.4344 × 0.045 mg) ÷ 2.467 mg] = 0.008 (99% lower confidence limit) < 0.1 (compliance maximum limit)
Since the declared value of 2.5 mg iron is compliant, the % DV declared must now be assessed.
- Assessment of declared % daily value (DV): For iron, the % DV is based on the rounded amount, by weight, of the nutrient, which is 2.5 mg declared.
- DV for iron as per Column 4 of Part 2 of the Table of daily values = 18 mg
- Rounded amount = 2.5 mg
(2.5 mg ÷ 18 mg) × 100 = 13.89 % is rounded to 14 % DV as per rounding rules (see Appendix 3, Table 1), which corresponds to the % DV declared, therefore compliant.
Note:
Also meets minimum level of 2.9 mg/100 g required for iron fortification of pasta.
5. Wieners - fat-reduced
Consider the example of wieners to assess the declaration in the NFt about a class II nutrient: "fat reduced"
- NFt declaration per 55 g serving: Fat = 7 g;
According to the Table - Sampling plan and tolerances, maximum pre-rounded values must be used for the assessment of fat.
- Laboratory results: fat-reduced product, sub-samples = 7.7 g, 8.2 g, 8.0 g; mean = 8.0 g
- Compliance limit criterion 1 for declared value:
(maximum pre-round + 50% of declared value) = [7.4 g + (0.5 × 7 g)] = 10.9 g - Compliance limit criterion 2 for declared value:
(maximum pre-round + 20% of declared value for class II) = [7.4 g + (0.2 × 7 g)] = 8.8 g (see Appendix 3, Table 3) - Assessment:
criterion 1: compliant for NFt: each sub-sample < 10.9 g
criterion 2: compliant for NFt: 8.0 g (mean) < 8.8 g
Nutrient content claim: fat reduced by 25% compared to regular brand Y wieners
- NFt declaration for Brand Y wieners: Fat = 10 g
- Laboratory results: regular Brand Y wieners, sub-samples = 10.3 g, 10.4 g, 10.5 g; mean = 10.4 g,
- Regulation: fat reduction claim - must be minimum 25% reduction
- Compliance limit, class II exception, comparative claims:
regular product: 10.4 g mean or if do not have laboratory analysis, 10 g declared, pre-rounded maximum = 10.4 g;
fat-reduced product: maximum limit @ 25% reduction = 0.75 × 10.4 g = 7.8 g
Assessment Class II exceptions, comparative claims: non-compliant for fat-reduction claim: 8.0 g (mean) > 7.8 g
6. Fruit drink - Vitamin C added
Consider the example of a fruit drink to assess the declaration in the NFt about a class I nutrient: Added vitamin C
- NFt declaration per 250 mL serving: Vitamin C = 60 mg
According to the Table - Sampling plan and tolerances, minimum pre-rounded values must be used for the assessment of vitamin C.
- Laboratory results: sub-samples = 50.0 mg, 85.2 mg, 100.2 mg; mean = 78.5 mg; s = 25.77 mg
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value) = [59.5 mg − (0.5 × 60 mg)] = 29.5 mg - Compliance limit criterion 2: Class I = pre-round minimum limit (no tolerance) = 59.5 mg (see Appendix 3, Table 2)
- Assessment:
criterion 1: compliant: each sub-sample > 29.5 mg
criterion 2: compliant: 78.5 mg (mean) > 59.5 mg
criterion 3: non-compliant: [(0.4344 × s) ÷ mean] = [(0.4344 × 25.77 mg) ÷ 78.5 mg] = 0.14 (99% lower confidence limit) > 0.1 (compliance maximum limit)
Part 2 - Analysis and feedback
Introduction and purpose
On January 1, 2003, Health Canada published amendments to the Food and Drug Regulations to require most prepackaged foods to bear a nutrition facts table, listing 13 nutrients and Calories, as well as updated regulations for nutrient content claims and disease risk reductions claims. These regulations aimed at providing Canadians with information to prevent injury to their health by helping them make appropriate food choices. However, since 2003, advances in science, changing Canadian consumption patterns, the increasing incidence and economic burden of chronic diseases, changing consumer expectations, and recent international actions (i.e. updates to the Nutrition Facts label in the U.S.) have necessitated updates to the Canadian Regulations on nutrition labelling. On December 14th 2016, Health Canada published, in Canada Gazette, Part II, amendments to the Food and Drug Regulations. These new requirements make the labelling information on prepackaged food products more useful and easier to read for Canadians.
The Canadian Food Inspection Agency (CFIA) is responsible for enforcing these food regulations of the Food and Drugs Act and Regulations. Health Canada and the Canadian Food Inspection Agency (CFIA) are committed to facilitating the implementation of these regulations in a manner that will retain the confidence of consumers and health professionals in the reliability of the nutrition information.
Industry is responsible for complying with the regulations and for ensuring the accuracy of label values. The regulations require that the nutrient values reflect the specific product being labelled. Given the many factors that affect the variability of nutrients in food and the high cost of laboratory analysis, the challenges for achieving accurate product specific information are recognized. In developing and/or verifying label values, industry may choose the risk management strategy best suited to the food(s) to be labelled, including the choice of sampling plans, methods of analysis, laboratories, whether to use a data base and the choice of data base and software.
The purpose of this Compliance Test is to provide a science based system for verifying the accuracy of nutrient values on labels and in advertising via laboratory analysis as part of assessing compliance with the Food and Drug Regulations. In January 2003, Health Canada introduced mandatory nutrition labelling, encompassing a wide variety of foods and an expanded list of nutrients, as well as the expansion of nutrient content claims and introduction of health claims. With these amendments, it was apparent that the then-existing system for testing accuracy of nutrient values needed review to achieve an improved, more effective system. This CFIA compliance test applies to nutrient data in the Nutrition Facts table, nutrient content claims and disease risk reduction claims, where they appear on labels and in advertising.
Information on procedures for generating label values is provided in the Guide to developing accurate nutrient values prepared by Health Canada. This will assist industry in developing their strategy, including sampling and analysis as well as using data bases/software packages to generate nutrient data for labels that are accurate, reliable and in compliance with the Food and Drug Regulations.
Background
Statutory authorities: Food and Drugs Act and Regulations
The CFIA is responsible for enforcing the food requirements of the Food and Drugs Act and Regulations. Verification of the accuracy of nutrient data on labels and in advertising is one part of the CFIA compliance strategy.
In Canada, there are no specific regulations dealing with tolerances and acceptance sampling to determine accuracy of nutrient amounts. Section 5 is the provision of the Food and Drugs Act relevant to truthful, accurate information.
Subsection 5(1) makes it an offence to "label, package . . . sell or advertise any food in a manner that is false, misleading or deceptive or is likely to create an erroneous impression regarding its character, value, quantity, composition, merit or safety".
Subsection 5(2) states that "an article of food that is not labelled or packaged as required by, or is labelled or packaged contrary to, the regulations shall be deemed to be labelled or packaged contrary to subsection (1)".
A food label or advertisement that bears nutrient values that are not truthful or accurate may be in violation of subsection 5(1), since it may be false and/or may create an erroneous impression as to the food's composition, merit or safety. In particular, an erroneous impression of safety may occur if the nutrient is one under strict control for the dietary management of disease. Under subsection 5(2), an inaccurate nutrition label may also be in violation of subsection 5(1) if it is considered to be labelled contrary to the requirements of the Food and Drug Regulations.
It is not technologically feasible or practical to determine with absolute accuracy the amount of nutrient in every container of food; a statistically sound sampling plan should, however, allow consumer confidence in the nutrition information and meet reasonable expectations of industry responsibility for accurately representing what is in the food, while respecting the inherent variability of nutrients in food and of manufacturing processes.
Compliance testing of declared nutrient values applies equally to imported and domestic product. Mutual recognition agreements and/or equivalence with exporting countries' measures will be pursued as appropriate to ensure that compliance with Canadian requirements does not unnecessarily impede trade.
History of assessing compliance of nutrient values in Canada
The procedure for evaluating compliance of nutrient levels in foods was first developed by the former Health and Welfare Canada in the 1980s for fortified foods. The procedure was designed using statistical theory of sampling, to provide reasonable assurance that unsatisfactory lots would not be overlooked (consumer's risk) and that satisfactory lots would not be falsely condemned (producer's risk). The procedure called for the selection of 5 containers at random from a lot, analysis of the nutrient concentration in each, and calculating the average of the 5 measurements. A 10% tolerance was allowed, for example, where a minimum requirement was stipulated, the lot would have been considered unsatisfactory if the average were less than 0.9 of the regulatory minimum. In addition, excessive variability was evaluated. A lot would have been considered unsatisfactory if any one sample were less than 0.3 of the regulatory minimum.
In 1990, following the introduction of voluntary nutrition labelling in Canada, Thompson and Jarvis, Health CanadaFootnote 4, added a second procedure to be used for the enforcement of nutrition labelling and claims by the former Consumer and Corporate Affairs Canada. The procedure called for 12 containers to be selected at random from a lot, and from these 12 containers a composite is formed and its nutrient composition analyzed. If a minimum were implied and if the resulting measurement were less than 0.8 of label declaration, the lot would be unsatisfactory. If a maximum were implied and if the resulting measurement were greater than 1.2 of the label value, the lot would also be considered unsatisfactory.
The rationale for using a single composite sample of 12 units for the naturally occurring nutrients was explained as follows: since the manufacturer does not always have control over the variability of the nutrient content from container to container, the sampling plan does not attempt to monitor variability.
A tolerance of 20% was allowed in recognition of the variability inherent in these nutrient concentrations, so that manufacturers would be encouraged to label a product with the true lot average. The analysis of a composite of 12 consumer units is the procedure used in the United States by the Food and Drug Administration (FDA) for verifying compliance of declared values in their nutrition facts table for naturally occurring nutrients.
After minor revisions resulting from comments received, it was published as part of the Guide to Food Labelling and Advertising in 1996. This revised procedure tightened requirements for claims to a 10% tolerance and provided no tolerance where a minimum or a maximum requirement is stipulated.
International situation
The Codex Alimentarius lists analytical methods for nutrients in the Codex Methods of Analysis and Sampling as well as general provisions for sampling plans for commodity standards in the Sampling Plans for Prepackaged FoodsFootnote 5.
In the US, the FDAFootnote 6 and the US Department of Agriculture (USDA) have, as part of the nutrition labelling rules, provisions for sampling, laboratory analysis and compliance limits for the analyzed value vs. label value, as well as provision for using nutrient data bases for the purpose of nutrition labelling. The Food Standards Australia New Zealand (FSANZ) provides for the use of nutrient values of national food and nutrient data bases for food labelling. Details of the international situation are given in Appendix 1.
Description of nutrition labelling compliance test
The nutrition labelling Compliance Test describes a set of conditions that constitutes the CFIA methodology for verifying nutrient accuracy of the Nutrition Facts table and claims set out in the regulations. The compliance test is not intended as a sampling plan for use by manufacturers. Manufacturers are responsible for determining the accuracy of nutrient values and may choose the method best suited to the food(s) to be labelled.
The Compliance Test comprises the laboratory analysis of the nutrient content of a statistically based sample from a lot and acceptance criteria. A minimum of 12 consumer units are to be randomly selected from a production or inspection lot of a food and arranged in three groups of four consumer units, with each group composited to provide three composite sub-samples for lab analysis. The principal acceptance criterion is a one-sided test for naturally occurring nutrients in nutrition labelling and claims, requiring accuracy within 20% of label values, i.e. the analyzed nutrient must be at least 80% of the label value for protein, carbohydrate, fibre, vitamins and minerals and not more than 120% of the label value for Calories, fat, saturated fat, trans fat, cholesterol, sugars and sodium for the nutrients in the Nutrition Facts table. These tolerances are adjusted for rounding rules, as applicable. Additional acceptance criteria respecting overall variability of nutrient content also apply.
For statements or claims respecting added vitamins, mineral nutrients and amino acids, the amount found in the sample must be at least equal to the label value.
Amounts may vary above labelled or required amounts where a minimum is stipulated and may vary below labelled or required amounts where a maximum is stipulated within good manufacturing practices.
Statistical framework
The Compliance Test is based on a sample of units selected from a lot. Basing a decision concerning a lot on a sample taken from that lot involves risk. The risk to the consumer is that a lot that does not meet the declared nutrient value will be judged satisfactory (consumer's risk); the risk to the producer is that a lot that does meet the declared nutrient value is judged unsatisfactory (producer's risk). A sampling plan is evaluated by estimating the magnitude of these two competing risks. Assumptions are made concerning the distribution of nutrients within and between lots and major sources of variation are considered.
Details of the statistical framework are set out in Appendix 2. Selected examples of the risks to consumers and producers of the compliance test are summarized in the tables below. The consumer's risk data suggest that for Class I nutrients (added vitamins and minerals, no tolerance), if the average concentration is only 90% of the declared nutrient value, nutrient variability (%CV) between 10% and 20%, test method variability (%CV) of 7%, there is a 3% to 7% chance that these products would be accepted. Producer's risk data for class I suggest that if the average concentration of added nutrients is 120% of label value, the chance that these products would be found non-compliant is between 0.2% - 1.5%. For class II nutrients where nutrient variability is typically higher, consumer's risk and producer's risk ranges are somewhat wider.
| Nutrient class | Criteria acceptance level |
True mean % label | Variability of nutrients within lot Table note e | Method variability Table note e | Between lab & lot variability Table note e | Consumer's risk (%) |
|---|---|---|---|---|---|---|
| Class I, added vitamins, minerals | ≥ 100%
declared nutrient value |
90% | 10% - 20% | 7% | 3% | 3% - 7% |
| Class II, protein, carbohydrate fibre, vitamins, minerals | ≥ 80%
declared nutrient value |
70 % | 10%-40% | 7% | 3% | 1% - 13% |
| Nutrient class | Criteria rejection level |
True mean % label value |
Variability of nutrients within lot Table note f | Method variability Table note f | Between lab & lot variability Table note f | Producer's risk (%) |
|---|---|---|---|---|---|---|
| Class I, added vitamins, minerals | < 100%
declared nutrient value |
120% | 10% - 20% | 7% | 3% | 0.2% -1.5% |
| Class II, protein, carbohydrate, fibre, vitamins, minerals | < 80%
declared nutrient value |
100% | 10% - 40% | 7% | 3% | 0.0% - 6% |
Nutrition labelling compliance test and rounding rules
The amendments to the Food and Drug Regulations include mandatory rounding rules for declared nutrient values. These regulations provide that an accepted range of values is represented by a single rounded value (see Appendix 3). Where determining the critical point for compliance purposes, both the accepted range of values before rounding and the compliance test tolerances must be factored into the calculation.
Criteria
A sound statistical framework forms the underpinning of the compliance test. In addition, the following criteria are consistent with CFIA and Health Canada policies and procedures and take into account stakeholder input; they were used to evaluate the CFIA Compliance Test:
1. Public health:
For consumers, label values that pass the Compliance Test should not increase risk to health.
Health risk could occur if the nutrient values on most food labels or on a label of a frequently consumed food differed consistently and by a significant amount from the actual concentration, and, as a result, a consumer mistakenly estimated the amount consumed. This could lead to over-consumption of calories and nutrients such as saturated and/or trans fat or under-consumption of nutrients, such as calcium over the long term. The main objective for the sampling procedure is to ensure that the sample taken is representative of the lot and measures, as closely as possible, the lot average while providing for some variability within the lot. Based on statistical analysis of all sources of variability involved, it is believed that, for most food products, values in the Nutrition Facts table, that pass the compliance test, would be close to the true mean. In addition, provision is made for using a more stringent tolerance on a case by case basis, if warranted, based on a health risk assessment.
2. Consumer protection:
For consumers, label values that pass the Compliance Test should, within reasonable limits of variability, accurately represent the nutrient content of the product purchased.
Taking into consideration the inherent variability of nutrients in food, the risk of erroneously accepting products of unacceptable quality with the proposed sampling plan is very low. Detail is presented in Appendix 2. For example, with an acceptance level of 80% of label value for the Nutrition Facts table, typical relative variability of the analytical method of 7%, and a relative nutrient variability of 10% to 40%, a lot of products with a true average of 70% of label value would have a 3%-17% chance of being erroneously accepted. Where the lot average value falls as low as 60% of label value, there is at most a 1% chance of being accepted. More stringent assessment criteria for comparisons were chosen, since, using a 20% tolerance combined with rounding, a comparison may not be valid if nutrient levels are highly variable. Therefore, the basis for assessment of comparative claims is that they be statistically justifiable. The evaluation of such claims usually involves the analysis of both the food for which the claim is made and the food to which it is compared.
3. Fair treatment of manufacturers:
For food processors accurately describing foods within reasonable limits of variability and following good manufacturing practices, label values should have a high probability of passing the Compliance Test.
The tolerances and sampling plan have taken into consideration nutrient content variability among containers within a lot, variability between different lots, as well as method variability and laboratory bias. Producers that use label values that take into account all of the above sources of variability should have a high probability of meeting the Compliance Test for all lots tested. For example, a 20% tolerance for nutrition labelling values as applied to a lot should allow foods to be labelled with representative values taking into account the natural variability of nutrients within the lot and variability in nutrient laboratory methods.
4. Effectiveness and efficiency of the inspection system:
The Compliance Test should be effective and efficient for industry to comply with and as a tool for government to use for the enforcement of the Food and Drug Regulations. The Compliance Test should be designed so as to fully achieve the objective without unnecessarily impeding trade.
The previous practice of using two different sampling plans (five or twelve units) and tolerances (10% and 20%) for nutrient content claims and nutrition labelling, respectively, has often been found to be inefficient. The analysis of 3 composite sub-samples of 4 consumer units each should be cost effective for the CFIA, since it would seem to be the minimum number of units and analyses needed for most situations in order to have confidence that the CFIA has not missed a non-compliant product and that an enforcement action is justified. Further, the Compliance Test should not unnecessarily impede trade, since, with few exceptions, the tolerances, sample number, rounding rules, definitions and laboratory methods are the same as those of the US, Canada's major trading partner.
Alternatives considered
It has been recognized for some time that a single analysis of a single container of food yields unreliable nutrient values. Health Canada, therefore, developed a policy to evaluate compliance based on a production lot and a sampling plan and tolerances that would take into account variations in nutrient composition and analytical measurements and that would minimize consumer's and producer's risk. Consequently, a statistically based system with the foregoing characteristics was the only approach considered; the choice of alternatives was among different sampling plans and acceptance criteria.
The three sampling plans considered as alternatives to the chosen sampling plan (a sample of 12 consumer units arranged in three groups of four units, composited) were already in use for evaluating nutrient content of a sample, as follows:
- one composite sample of 12 consumer units, used previously to assess the accuracy of nutrition labelling for naturally occurring nutrients, also used by the FDA
- one composite sample of six consumer units, used by the USDA to assess the accuracy of nutrition information for meat and poultry, and
- five consumer units, analyzed individually, applied by CFIA to fortified foods (added vitamins, mineral nutrients and amino acids)
The producer's risk and consumer's risk for these three alternative systems incorporating different levels of variation in composition and analytical reproducibility were assessed. These data show that the producer's risk and consumer's risk are generally lowest with the chosen scheme (scenario A - three composite samples of four consumer units). Although any one of the alternative schemes would have met most of the criteria established, there were specific reasons for rejecting each one, as follows:
- one composite of 12 consumer units (scenario B) was rejected, since it did not include provision for measuring variability within the lot; however, the selection of 12 consumer units was retained in the proposal to facilitate equivalence with the US
- one composite of six consumer units (scenario C) although harmonized with that of the USDA (meat only), was found to result in slightly higher risk profiles. Also, it was preferred to choose a single sampling procedure for all products
- similarly, analysis of five individual samples (scenario D) was rejected, since it did not conform to the 12 unit sample scheme, and the analysis of five individual units was more expensive
The following table provides additional information on the relative strengths and weaknesses of the four scenarios considered:
| Scenario A (Compliance Test) 12 units 3 composites 4 |
Scenario B 12 units 1 composite |
Scenario C 6 units 1 composite |
Scenario D 5 units individual |
|
|---|---|---|---|---|
| Consumer's risk within lot nutrient variability Table note g < 20%, method variability Table note g 7% |
lowest consumer's risk | 3rd lowest risk | 4th lowest risk | 2nd lowest risk |
| Producer's risk within lot nutrient variability Table Note g < 20% method variability Table Note g 7% |
lowest producer's risk | 3rd lowest risk | 4th lowest risk | 2nd lowest risk |
| Measures variability within lot | yes | no | no | yes |
| CFIA cost of sample pickup & analysis | 2nd highest | 3rd highest | 4th highest | highest |
| Compatibility with US system | same no. units as US FDA, compatible with USDA | same as US FDA | same as USDA | fewer units than FDA and USDA |
A difference between the FDA procedure (Scenario B) and the CFIA procedure (Scenario A) is that the US procedure makes allowance for the variability of the analytical method if it exceeds the tolerances. The Canadian procedure includes the variability of the analytical method in the tolerances.
The government's development of representative label values for single ingredient foods for use directly on labels by producers and retailers was considered. Pre-approval of industry data bases for nutrition labelling was also considered. However, it was decided that, before the end of the transition period, industry would be encouraged to develop nutrient data sources and related software and analytical capacity that are needed to generate the Nutrition Facts table. Health Canada would assist in providing guidance and expertise.
Feedback
In response to Health Canada's policy proposals, published in October, 2000 and later proposed regulations in Canada Gazette, Part I, June 16, 2001, many comments were received on compliance of nutrient values on labels and related enforcement issues. Meetings were held with producer groups and manufacturers on nutrient data needs, use of published data and compliance policy respecting nutrient accuracy. Stakeholders commented as follows:
Consumer and health organizations:
- emphasized the importance of ensuring accuracy and reliability of label information
- supported a clear enforcement policy, a standardized process for verification of data and a strong monitoring effort to ensure compliance and public confidence in the new label
- recommended that the nutrition label value not vary more than 10% from the nutrient value determined in the sample by analysis
- supported consistency between companies marketing identical foods to avoid consumer confusion
- favoured updating and expanding the Canadian Nutrient File with government leadership and food industry involvement
Industry
- favoured reasonable tolerance limits for accuracy of declared nutrients, such as a 20% limit, except where nutrient values are very small
- favoured system for assessing accuracy equivalent to that of the United States
- considered a 10% tolerance difficult to meet due to limitations in analytical accuracy, variability in manufacturing, added nutrients and variations in nutrient content of ingredients depending on the variety and season
- supported the use of data bases and computer software to generate nutrition data to help reduce the economic burden and improve consistency
- recommended that Health Canada review nutrient data generated for labelling purposes
- recommended that government work collaboratively with the industry to develop nutrient data bases and/or Canadian Reference Tables for raw single-ingredient commodities
- favoured updating the Canadian Nutrient File
Feedback to the consultation document
Interested parties were invited to comment within 45 days to a CFIA Consultation Document on a proposed compliance test, issued November 28, 2002. Responses were received from sixteen stakeholders, including the Consumers' Association of Canada, eight major national associations representing either producers, manufacturers or retailers, a number of individual manufacturers, a commercial laboratory and a university professor of nutritional sciences. The respondents generally supported the overall approach and the sampling plan. The majority supported 20% tolerance limits for the Nutrition Facts table. The need for nationally representative nutrient data for raw single ingredient foods, whose labelling is voluntary, was reiterated. Responses to specific questions are as follows with the CFIA position in bold:
General
Q1. Do you have comments on the approach to minimizing the consumer's risk and producer's risk in developing a standardized compliance test for verifying product specific nutrient data on labels?
There was general agreement with the proposed approach for the compliance test, i.e. to seek to minimize the consumer's risk and producer's risk. A few expressed reservations, stating that the approach was not useful for raw and/or wild single ingredient foods, which tend to have large seasonal and lot-to-lot variation. However, CFIA notes that since consumers and further processors need reliable nutrient content information, it may be appropriate for these foods to have two sets of labels to more accurately reflect the nutrient value of the product, or alternatively, one label using conservative nutrient values.
The proposed approach, to seek to minimize the consumer's risk and producer's risk, is maintained.
Definition of a lot
Q1. Do you agree with the definition of a lot? Why or why not?
Concern was expressed that the definition of a "lot" needed to be more concise, also that the definition was not well suited to import and retail situations.
The definition of a lot has therefore been revised to more concisely reflect the underlying principle of uniformity when selecting samples.
Sampling
Q1. Do you agree with taking a sample of 12 consumer units from a lot, as in the US, to verify compliance? Why or Why not?
Q2. Do you agree with grouping consumer units in three sub-samples of four for laboratory analysis? Why or Why not?
There was general agreement with the proposed sampling plan. One respondent suggested that the sample be changed to 2 composites of 6 units, as it would be less costly for processors. While internal analysis has shown that 2 of 6 units is less costly, 3 of 4 results in lower producer's risk and consumer's risk. CFIA notes that processors are not compelled to adopt the sampling plan used by the CFIA, and can develop their own sampling plans as appropriate.
The proposed approach to take 12 consumer units from a lot, then group them into three sub-samples of four for laboratory analysis is therefore adopted.
Tolerances
Q1. Given that the tolerances must be applied to the pre-rounded values, resulting in a larger overall tolerance that is variable, is the 20% tolerance for the Nutrition Facts table appropriate?
There was general agreement with the proposal with a few exceptions. One industry representative suggested a tighter tolerance of 15%. However, a consumer group suggested that a range of values be acceptable for raw single ingredient food, especially fruits and vegetables, where the inherent nutrient variability is wide. However, based on CFIA's statistical analysis and experience, a 20% tolerance is sufficiently generous to allow an average value for a lot to be in compliance most of the time (e.g. a 6% risk of food being found out of compliance, if CV of nutrient distribution is less than 40% and method variability is 7%).
The proposed approach to apply a 20% tolerance to the nutrient values declared in the Nutrient Facts table is therefore adopted.
Q2. Do you agree that there should be a tighter tolerance for nutrient values that are the subject of claims (10%) than those in the Nutrition Facts table? Why or Why not?
While several agreed that companies making claims should be held to a higher level of accuracy, most did not agree fully with the proposal for a tighter (10%) cut-off for claims than for the Nutrition Facts table. Concern was expressed that a tighter tolerance for nutrient values that are the subject of claims, particularly in relation to certain "low" and "free" claims, would be more difficult to achieve. Several believed that tolerances for claims and nutrition labelling should be the same, since both are "commitments" under the law, the integrity of the labelling process was being judged and that consumers value other nutrients as well as those claimed when judging products. One association found dealing with several tolerances cumbersome. Another mentioned the difficulties for small businesses and single ingredient foods. The CFIA found these arguments persuasive. In addition, similar treatment of claims and nutrition labelling is consistent with the US approach and the adoption of the same approach in Canada would facilitate mutual equivalence with the US.
The tolerance for analyzed nutrient values that are the subject of a nutrient content claim is therefore revised from the proposed 10% to 20% (80%/120%), the same as for the Nutrition Facts table, with exceptions for a health risk and a comparative claim.
Q3. Do you agree that there should be no tolerance for minimum or maximum requirement for claims e.g. in the case of a low fat claims, the sample may not exceed 3 g per serving? Why or Why not?
There was some support for the proposal, but also a number of objections, similar to those in response to the 10% tolerance. Concern was expressed that allowing no tolerance for minimum and maximum requirements for nutrient values that are the subject of claims would be difficult to achieve, particularly in relation to certain "low" and "free" claims.
Therefore the proposed approach is revised to apply a 20% tolerance to analyzed nutrient values in relation to a minimum and maximum requirement for a nutrient content claim.
Q4. Do you agree that there should be no tolerances for declaration of added nutrients? Why or why not?
Support for the proposal was mixed. Several respondents agreed, stating that nutrient ingredients could be more readily controlled than indigenous nutrients and that manufacturers are selling a benefit that the consumer expects to get and should be held to the amounts claimed. Those adding nutrients expressed the need for a tolerance, due to the variation in the naturally occurring nutrients whose amount in the food is combined with the amount of added nutrient for the label value and the higher analytical variability for vitamins. However, Health Canada is of the opinion that levels should not fall below the declared amount of a vitamin or mineral nutrient that is added. Further, this position is consistent with that of the United States.
As a result, no tolerance for the declaration of added nutrients will be allowed.
Q5. Should there be an allowance for method variability, only, in the case of (Q3) and (Q4)? Why or why not?
Q6. Do you agree that the tolerances should incorporate the variability of the method, rather than be additional to the method variability allowance?
Industry stakeholders supported an additional allowance for method variability in all cases. However, CFIA notes that estimates for consumer's risk and producer's risk include method variability as part of the total variability. Further tolerance provisions for method variability would increase consumer's risk. The CFIA will analyze 3 sub-samples of 4 units, which will reduce method variability to 1/3 of the method variability for a single analysis. As noted above, tolerances have been increased to 20% for claims (Q3).
Due to the foregoing and since the rounding rules already contribute additional consumer risk, allowance for method variability will only be considered for label values of added nutrients.
Acceptance criteria relating to variability
Q1. Do you consider the acceptance criteria relating to variability (criteria 1 and 3) reasonable? Why or why not?
Q2. Do you agree that these criteria should only be applied to added nutrients or in the case of claims? Why or why not?
Not many stakeholders had an opinion on this issue. Of the five commenting, three supported Criteria 1 and 3. However, they would apply variability criteria to nutrition labelling as well as to claims and added nutrients. While the CFIA agrees that variability of nutrient distribution within a lot should not be excessive, the inherent variability of naturally occurring nutrients in foods is recognized. Added vitamins and minerals are more readily controlled and there may be safety issues associated with large variability.
Therefore, the proposed criteria 1 and 3 will be retained. Criterion 3 will be applied to class 1, added nutrients, only.
Variations "within good manufacturing practices"
Q1. Do you agree that values may vary "within good manufacturing practices" over labelled amounts in the case of a minimum requirement or may vary under labelled amounts "within good manufacturing practices" in the case of a maximum requirement?
Q2. What would be a reasonable excess or deficiency? For example, if the vitamin content of a sample must be at least 80% of declared amount, how much variation above the declared amount is reasonable?
Most agreed with the proposal that nutrient values with a minimum requirement, may exceed labelled amounts "within good manufacturing practices" and that nutrient values with a maximum requirement may be less than labelled amounts "within good manufacturing practices". One-sided tolerances were considered appropriate in circumstances where health concerns are relevant primarily to over-consumption or to under-consumption, because they ensure that the claimed amount of the nutrient is present in the food without unreasonably varying beyond what is declared to ensure the declared level is present. A few did not favour one-sided tolerances. It was suggested that there should be upper limits for carbohydrate values, of concern to consumers with diabetes, as well as upper limits for protein values, of concern to those following a special diet low in protein. Also, there was concern that the one-sided tolerances would lead to misleading labels. An industry representative suggested a range between 80% and 120% of the label amount. The CFIA acknowledges the merits of a two-sided approach, i.e. an acceptable range of 80%-120% of the label amount; however, since this range may be difficult to achieve for many manufacturers, a one-sided approach, either minimum or maximum provides the greatest health benefit.
Therefore, the proposed approach to allow nutrient values with a minimum requirement to exceed the label value "within good manufacturing practices" and those with a maximum requirement to be less than labelled amounts "within good manufacturing practices" will be retained with the proviso that there is no risk to health.
Other comments:
Databases
Similar to responses to previous Health Canada consultations on nutrition labelling, a consumer association, as well as producer and retail associations expressed disappointment that development of nationally representative nutrient data for raw single ingredient foods and their acceptance for the purpose of nutrition labelling to provide consistency of values for consumers, was not pursued. It was noted that consumers want relative information for those foods not covered by mandatory nutrition labelling as they provide significant contributions to nutritional intakes.
Definitive compliance will be based on laboratory analysis. Evaluation of data bases is not part of the compliance test. However, a nutrient data base may be used as a tool in developing nutrition labelling values; consideration of data sources is part of the compliance approach and would be incorporated in the compliance assessment tool for nutrition labelling, being developed for use in establishment inspection. Information on procedures for generating label values are provided in the Guide to developing accurate nutrient values prepared by Health Canada.
References
Appendix 1 - International context
1. Codex Alimentarius
Codex guidelines on nutrition labelling
The Codex Alimentarius Guidelines on Nutrition Labelling (reference CAC/GL 2-1985) set out the nutrients to be declared where nutrition labelling is applied. Dietary fibre, sugars and polyunsaturated fatty acids are defined and the calculation of energy and protein described. Available carbohydrate is declared as "carbohydrates".
The Codex guidelines recommend that tolerance limits be set in relation to public health concerns, shelf-life, accuracy of analysis, processing variability and inherent lability and variability of the nutrient in the product, and according to whether the nutrient has been added or is naturally occurring in the product.
The guidelines also recommend weighted average values derived from data specifically obtained from analyses of products which are representative of the product being labelled.
Finally, the guidelines state that, in those cases where a product is subject to a Codex standard, requirements for tolerances for nutrient declaration established by the standard should take precedence over these guidelines.
The 1989 Codex report on laboratory analysis and sampling of nutrients supported the testing of composite samples using AOAC methods.
Codex methods of analysis and sampling
The FAO/WHO Codex Alimentarius Recommended Methods of Analysis and Sampling (reference CODEX STAN 234-1999) includes methods for the determination of carbohydrate, protein, fat, polyunsaturated fat, saturated fat and many other nutrients, listed under the Guidelines on Nutrition Labelling or Foods For Special Dietary Use. The Codex Sampling Plan for Prepackaged Foods, 1969, is intended to cover quality requirements in Codex commodity standards. Sample numbers are based on the size of the lot. An Acceptable Quality Level (AQL) would be used for rejecting an inspected lot. For many Codex commodity standards, the sampling plan would accept a lot that has 6.5% defective units approximately 95% of the time.
2. Australia and New Zealand
The Food Standards Code of Food Standards Australia New Zealand (FSANZ) provides for the declaration of an average quantity of a substance that may be determined from one or more of the following:
- the manufacturer's analysis of the food
- calculation from the actual or average quantity of nutrients in the ingredients used
- calculation from generally accepted data
In addition FSANZ provides a Nutrition Panel Calculator to allow manufacturers to calculate nutrients using the Australian Food and Nutrient Database. The Australian Food and Nutrient Database is supported by FSANZ and contains nutrient data on 4,500 foods sold in Australia.
3. European Union - European Commission
The Council Directive on nutrition labelling for foodstuffs of the Council of European Communities 90/496/EEC includes the following provisions:
"Average value" means the value which best represents the amount of the nutrient which a given food contains, and reflects allowances for seasonal variability, patterns of consumption and other factors which may cause the actual value to vary.
The declared values shall, according to the individual case, be average values based on:
- the manufacturer's analysis of the food
- a calculation from the known or actual average value of the ingredients used
- a calculation from generally established and accepted data
The rules for implementing paragraph (a) with regard to differences between declared values and those established in the course of official checks are decided upon in accordance with a procedure laid down in the Directive.
4. The United States of America
The United States (US) Food and Drug Administration (FDA) and Department of Agriculture (USDA) published final rules codifying the Nutrition Labelling and Education Act (NLEA) in 1993. These rules include compliance provisions for determining accuracy of declared nutrients and setting out methodology for sampling, analysis and tolerances. They also included provisions for use of databases.
The sample for nutrient analysis must consist of a composite of a minimum of 12 consumer units (FDA) or a composite of a minimum of 6 consumer units or the average of 6 individual units (USDA) taken one from each of 12 different randomly chosen shipping cases, to be representative of a production lot.
Methods of analysis are:
- FDA - the appropriate method in the official methods of AOAC International
- USDA - the appropriate USDA method in the Chemistry Laboratory Guidebook or if no method exists in the Guidebook, the appropriate AOAC method
For class I nutrients, i.e. added vitamin, mineral, protein, dietary fibre or potassium in fortified or fabricated foods, the nutrient content of the composite sample must be at least equal to the value declared on the label.
For class II, i.e., naturally occurring (indigenous) nutrients, class II vitamin, mineral, protein, total carbohydrate, other carbohydrate, polyunsaturated and mono-unsaturated fat or potassium, the nutrient content of the composite must be least equal to 80% of the value for that nutrient declared on the label. The nutrient content of a food with a label declaration of calories, sugars, total fat, saturated fat, cholesterol, or sodium shall not be greater than 20% in excess of the value for that nutrient declared on the label. In addition, the provisions state that no regulatory action will be based on a determination of a nutrient value that falls beyond these levels by an amount less than the variability generally recognized for the analytical method used in that food at the level involved and in the case of USDA, less than the inherent nutrient variation in the product.
The provisions state that reasonable excesses of a vitamin, mineral, protein, total carbohydrate, dietary fibre, other carbohydrate, polyunsaturated or mono-unsaturated fat or potassium over labelled amounts are acceptable within good manufacturing practice. Reasonable deficiencies of calories, sugars, total fat, saturated fat, cholesterol, or sodium under labelled amounts are acceptable within good manufacturing practices.
Provisions also allow for the use of a nutrient database as follows: in the case of FDA, compliance with the above provisions may be provided by use of an FDA approved database that has been computed following FDA guideline procedures and where food samples have been handled in accordance with current good manufacturing practice to prevent nutrient loss; in the case of USDA, for single ingredient raw meat and poultry (including ground beef) products, nutrition labelling is based on the most current representative database values (average values) contained in USDA's National Nutrient Data Bank.
Appendix 2 - Statistical framework
Background
Nutrition values on labels and in advertising need to be accurate and measurable for compliance purposes. Consequently, the food industry seeks a high probability of passing a nutrition labelling compliance test while the consumer requires a high probability that the label value accurately reflects the nutrient content of the product. Balancing these objectives is key to the development and acceptability of a compliance test. To achieve this balance, one needs to consider, on the one hand, the likelihood that a lot meeting the label declaration will be deemed unacceptable and, on the other, the likelihood that a lot not meeting the label declaration will be judged satisfactory. In statistical terminology, this translates to a type I error, or producer's risk, which is the probability that a lot of acceptable declared values is erroneously rejected, and a type II error, or consumer's risk, which is the probability that a lot of unacceptable declared values is erroneously accepted as satisfactory. A statistically valid and defensible sampling plan then needs to consider the relative magnitude and ensuing consequences of these two competing errors. Moreover, not only does the mean nutrient content of the food need to be evaluated by the sampling scheme but equally important is its capacity to include and measure the associated variability.
A. Sampling plan
To meet all these requirements, a number of assumptions, some based on past experience and observation, need to be made to enable the computation of appropriate type I and type II errors corresponding to a specific sampling plan. The sampling scheme adopted by the CFIA for assessing the accuracy of nutrition labelling involves the selection of twelve individual random units from a lot, arranged in three groups of four units, with each group properly composited to provide three composite sub-samples for lab analysis. The accompanying tables provide the risk/error information for this sampling regime under the following assumptions.
Assumptions
- All samples are randomly selected from a lot to ensure representativeness of the lot and its characteristics and to allow statistical inference from the lab results.
- Let S represent the variability among the units within a lot
A represent the variability among the mean nutrient values of various lots
R represent the measurement variability within a laboratory, namely, the repeatability variance
B represent the measurement variability between laboratories
b represent the number of lots (or batches) sampled
c represent the number of composite sub-samples analyzed per lot
d represent the number of individual units from the lot comprising a composite sub-sample.
Then the total variance of a measurement for d units in each of c analyses from b lots is given as
- In all of the estimates, the nutrients are assumed to have an underlying normal distribution with mean µ and variance
 (or S). This implies that the underlying coefficient of variation (CV) is less than 50%
(or S). This implies that the underlying coefficient of variation (CV) is less than 50% - The lot-to-lot and lab-to-lab variability (A and B) are assumed independent and their combined relative standard deviation
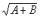 is historically accepted as 3%. Nonetheless, we have also investigated this model assumption for 7% in the accompanying tables.
is historically accepted as 3%. Nonetheless, we have also investigated this model assumption for 7% in the accompanying tables.
Note: In the following tables, the results are given for three different values of the measurement variation within a laboratory, namely the repeatability relative standard deviation (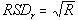 ), expressed as a coefficient of variation, specifically, 3%, 7% and 15%.
It should be realized that, as this variability increases, the only way of reducing errors requires that one analyze individual rather than composite samples from the lot
), expressed as a coefficient of variation, specifically, 3%, 7% and 15%.
It should be realized that, as this variability increases, the only way of reducing errors requires that one analyze individual rather than composite samples from the lot
Tables 1 - 3
Tables 1 - 3 show how the test method variability and the variability of the nutrient distribution in a food impact on the producer's risk and the consumer's risk for different average lot quantities (true mean as % of label). Three tables, one for each of class I, class II (minimum limit) and class II (maximum limit) are presented. Within each table are four individual tables, two for producer's risk and two for consumer's risk, and each of these is shown for 3% CV and 7% CV lab-to -lab and lot-to- lot variability respectively. Then, for each individual table, the producer's risk or consumer's risk is shown in relation to the variability of the analytical method within a lab (RSDr%) and variability of the nutrient distribution within a lot (CV%).
Using the relevant table, the producer's risk or chance that a lot of a given mean nutrient content would be erroneously rejected (found out of compliance) and consumer's risk or chance that the lot would be erroneously accepted (found compliant) can be estimated if the nutrient variability (CV%) and method variability lab (RSDr%) are known. For example, in Table 1, class I, added vitamins and mineral nutrients, the producer's risk of a lot being erroneously rejected would be 5.9%, where the true average nutrient content of the lot is 110% of the label, a test RSDr% of 7% and a lot nutrient variability CV of 10%. The consumer's risk or chance that a lot of average nutrient content of 90% of label would be erroneously accepted would be 2.8% for the same test variability and within lot nutrient distribution.
In Table 3, class II maximum limit (Calories, fat, saturated fat, trans fat, cholesterol, sugars and sodium), for a test variability of 7% and a CV of nutrient distribution of 20%, if the true lot average is 100% of label, the chance of erroneously rejecting a lot would be 0.5% (producer's risk); and under the same conditions of test variability and nutrient variability, a true lot average of 140% of the label value, there would be a 3.1% chance of accepting the products erroneously (consumer's risk).
Table 1
Producer's risk and consumer's risk for class I
Added vitamins and mineral nutrients
The sample is comprised of 12 consumer units taken at random from a lot arranged in three composites of four units.
The mean nutrient content of the sample is not less than the declared label value (adjusted for rounding)
| Between lot & between lab variability CV = 3% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr Table note h (%) |
Variability of nutrients within lot (CV Table note i of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 110 | 3 | 2.2 | 8.8 | 16.5 | 22.5 | 27.0 |
| 7 | 5.9 | 11.8 | 18.2 | 23.5 | 27.6 | |
| 15 | 17.2 | 20.1 | 23.5 | 26.9 | 29.7 | |
| 120 | 3 | 0.0 | 0.7 | 3.7 | 8.3 | 13.1 |
| 7 | 0.2 | 1.5 | 4.8 | 9.3 | 13.8 | |
| 15 | 4.1 | 6.2 | 9.3 | 12.9 | 16.5 | |
| 130 | 3 | 0.0 | 0.0 | 0.7 | 2.8 | 6.0 |
| 7 | 0.0 | 0.1 | 1.1 | 3.3 | 6.6 | |
| 15 | 0.8 | 1.7 | 3.4 | 5.9 | 8.9 | |
| 140 | 3 | 0.0 | 0.0 | 0.1 | 0.9 | 2.7 |
| 7 | 0.0 | 0.0 | 0.2 | 1.2 | 3.1 | |
| 15 | 0.10 | 0.40 | 1.2 | 2.6 | 4.7 | |
| Between lot & between lab variability CV = 7% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 110 | 3 | 12.1 | 16.3 | 21.0 | 25.2 | 28.7 |
| 7 | 14.5 | 18.0 | 22.1 | 25.9 | 29.1 | |
| 15 | 21.5 | 23.4 | 26.0 | 28.5 | 30.9 | |
| 120 | 3 | 1.6 | 3.6 | 7.0 | 11.0 | 15.1 |
| 7 | 2.6 | 4.7 | 8.0 | 11.9 | 15.7 | |
| 15 | 7.4 | 9.2 | 11.9 | 14.9 | 18.0 | |
| 130 | 3 | 0.1 | 0.6 | 2.0 | 4.5 | 7.6 |
| 7 | 0.4 | 1.0 | 2.6 | 5.1 | 8.2 | |
| 15 | 2.2 | 3.3 | 5.1 | 7.5 | 10.3 | |
| 140 | 3 | 0.0 | 0.1 | 0.6 | 1.8 | 3.8 |
| 7 | 0.0 | 0.2 | 0.8 | 2.1 | 4.2 | |
| 15 | 0.7 | 1.1 | 2.1 | 3.7 | 5.9 | |
| Between lot & between lab variability CV = 3% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV Table note j of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 95 | 3 | 12.2 | 21.7 | 28.6 | 33.1 | 36.1 |
| 7 | 18.2 | 24.6 | 30.0 | 33.8 | 36.5 | |
| 15 | 29.2 | 31.4 | 33.8 | 36.1 | 37.9 | |
| 90 | 3 | 0.7 | 4.9 | 11.7 | 17.8 | 22.7 |
| 7 | 2.8 | 7.3 | 13.4 | 18.9 | 23.4 | |
| 15 | 12.4 | 15.3 | 18.9 | 22.6 | 25.8 | |
| 80 | 3 | 0.0 | 0.0 | 0.4 | 1.9 | 4.6 |
| 7 | 0.0 | 0.1 | 0.6 | 2.4 | 5.1 | |
| 15 | 0.5 | 1.1 | 2.4 | 4.5 | 7.2 | |
| 70 | 3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| 7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | |
| 15 | 0.0 | 0.0 | 0.0 | 0.2 | 0.6 | |
| Between lot & between lab variability CV = 7% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 95 | 3 | 24.9 | 28.4 | 32.0 | 35.0 | 37.2 |
| 7 | 27.0 | 29.8 | 32.8 | 35.4 | 37.5 | |
| 15 | 32.4 | 33.7 | 35.5 | 37.1 | 38.6 | |
| 90 | 3 | 7.6 | 11.5 | 16.2 | 20.7 | 24.6 |
| 7 | 9.8 | 13.2 | 17.4 | 21.5 | 25.1 | |
| 15 | 16.7 | 18.8 | 21.5 | 24.4 | 27.1 | |
| 80 | 3 | 0.1 | 0.3 | 1.3 | 3.3 | 6.1 |
| 7 | 0.2 | 0.6 | 1.7 | 3.8 | 6.5 | |
| 15 | 1.5 | 2.3 | 3.8 | 6.0 | 8.5 | |
| 70 | 3 | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 |
| 7 | 0.0 | 0.0 | 0.0 | 0.1 | 0.5 | |
| 15 | 0.0 | 0.0 | 0.1 | 0.4 | 0.9 | |
Table 2
Producer's risk and consumer's risk for class II minimum limit
Protein, carbohydrate, fibre, vitamins, mineral nutrients
The sample is comprised of 12 consumer units taken at random from a lot arranged in three composites of four units.
The mean nutrient content of the sample is not less than 80% of the declared label value (adjusted for rounding).
| Between lot & between lab variability CV = 3% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr Table note k (%) |
Variability of nutrients within lot (CV Table note l of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 90 | 3 | 0.7 | 4.9 | 11.7 | 17.8 | 22.7 |
| 7 | 2.8 | 7.3 | 13.4 | 18.9 | 23.4 | |
| 15 | 12.4 | 15.3 | 18.9 | 22.6 | 25.8 | |
| 100 | 3 | 0.0 | 0.1 | 1.6 | 4.9 | 8.9 |
| 7 | 0.0 | 0.5 | 2.3 | 5.6 | 9.5 | |
| 15 | 1.9 | 3.2 | 5.6 | 8.7 | 12.1 | |
| 110 | 3 | 0.0 | 0.0 | 0.2 | 1.2 | 3.3 |
| 7 | 0.0 | 0.0 | 0.3 | 1.5 | 3.7 | |
| 15 | 0.2 | 0.6 | 1.5 | 3.2 | 5.5 | |
| 120 | 3 | 0.0 | 0.0 | 0.0 | 0.3 | 1.2 |
| 7 | 0.0 | 0.0 | 0.0 | 0.4 | 1.5 | |
| 15 | 0.0 | 0.1 | 0.4 | 1.2 | 2.6 | |
| Between lot & between lab variability CV = 7% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 90 | 3 | 7.6 | 11.5 | 16.2 | 20.7 | 24.6 |
| 7 | 9.8 | 13.2 | 17.4 | 21.5 | 25.1 | |
| 15 | 16.7 | 18.8 | 21.5 | 24.4 | 27.1 | |
| 100 | 3 | 0.5 | 1.5 | 3.8 | 7.1 | 10.8 |
| 7 | 1.0 | 2.2 | 4.6 | 7.8 | 11.3 | |
| 15 | 4.1 | 5.5 | 7.8 | 10.6 | 13.6 | |
| 110 | 3 | 0.0 | 0.2 | 0.8 | 2.3 | 4.5 |
| 7 | 0.1 | 0.3 | 1.1 | 2.6 | 5.0 | |
| 15 | 0.9 | 1.5 | 2.7 | 4.5 | 6.7 | |
| 120 | 3 | 0.0 | 0.0 | 0.2 | 0.7 | 1.9 |
| 7 | 0.0 | 0.0 | 0.2 | 0.9 | 2.2 | |
| 15 | 0.2 | 0.4 | 0.9 | 1.9 | 3.4 | |
| Between lot & between lab variability CV = 3% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true Mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 75 | 3 | 7.0 | 16.1 | 23.7 | 29.0 | 32.7 |
| 7 | 12.5 | 19.2 | 25.3 | 29.8 | 33.1 | |
| 15 | 24.4 | 26.9 | 29.9 | 32.6 | 34.8 | |
| 70 | 3 | 0.1 | 1.7 | 6.3 | 11.8 | 16.8 |
| 7 | 0.7 | 3.1 | 7.7 | 12.8 | 17.5 | |
| 15 | 6.9 | 9.4 | 12.9 | 16.6 | 20.2 | |
| 60 | 3 | 0.0 | 0.0 | 0.0 | 0.3 | 1.2 |
| 7 | 0.0 | 0.0 | 0.0 | 0.4 | 1.5 | |
| 15 | 0.0 | 0.1 | 0.4 | 1.2 | 2.6 | |
| Between lot & between lab variability CV = 7% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 75 | 3 | 19.5 | 23.5 | 27.7 | 31.2 | 34.0 |
| 7 | 21.9 | 25.1 | 28.7 | 31.8 | 34.3 | |
| 15 | 28.1 | 29.8 | 31.8 | 33.9 | 35.7 | |
| 70 | 3 | 3.3 | 6.1 | 10.2 | 14.7 | 18.8 |
| 7 | 4.8 | 7.5 | 11.4 | 15.5 | 19.4 | |
| 15 | 10.7 | 12.7 | 15.6 | 18.7 | 21.7 | |
| 60 | 3 | 0.0 | 0.0 | 0.2 | 0.7 | 1.9 |
| 7 | 0.0 | 0.0 | 0.2 | 0.9 | 2.2 | |
| 15 | 0.2 | 0.4 | 0.9 | 1.9 | 3.4 | |
Table 3
Producer's risk and consumer's risk for class II maximum limit
Calories, fat, saturated fat, trans fat, sugars, sodium
The sample is comprised of 12 consumer units taken at random from a lot arranged in three composites of four units.
The mean nutrient content of the sample is not more than 120% of the declared label value (adjusted for rounding).
| Between lot & between lab variability CV = 3% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr Table note m (%) |
Variability of nutrients within lot (CV Table note n of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 110 | 3 | 2.2 | 8.8 | 16.5 | 22.5 | 27.0 |
| 7 | 5.9 | 11.8 | 18.2 | 23.5 | 27.6 | |
| 15 | 17.2 | 20.1 | 23.5 | 26.9 | 29.7 | |
| 100 | 3 | 0.0 | 0.1 | 1.6 | 4.9 | 8.9 |
| 7 | 0.0 | 0.5 | 2.3 | 5.6 | 9.5 | |
| 15 | 1.9 | 3.2 | 5.6 | 8.7 | 12.1 | |
| 90 | 3 | 0.0 | 0.0 | 0.0 | 0.3 | 1.2 |
| 7 | 0.0 | 0.0 | 0.0 | 0.4 | 1.5 | |
| 15 | 0.0 | 0.1 | 0.4 | 1.2 | 2.6 | |
| Between lot & between lab variability CV = 7% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 110 | 3 | 12.1 | 16.3 | 21.0 | 25.2 | 28.7 |
| 7 | 14.5 | 18.0 | 22.1 | 25.9 | 29.1 | |
| 15 | 21.5 | 23.4 | 26.0 | 28.5 | 30.9 | |
| 100 | 3 | 0.5 | 1.5 | 3.8 | 7.1 | 10.8 |
| 7 | 0.0 | 2.2 | 4.6 | 7.8 | 11.3 | |
| 15 | 0.0 | 5.5 | 7.8 | 10.6 | 13.6 | |
| 90 | 3 | 0.0 | 0.0 | 0.2 | 0.7 | 1.9 |
| 7 | 0.0 | 0.0 | 0.2 | 0.9 | 2.2 | |
| 15 | 0.0 | 0.4 | 0.9 | 1.9 | 3.4 | |
| Between lot & between lab variability CV = 3% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 125 | 3 | 18.8 | 27.6 | 33.4 | 37.0 | 39.4 |
| 7 | 24.5 | 30.1 | 34.5 | 37.5 | 39.7 | |
| 15 | 33.9 | 35.6 | 37.6 | 39.3 | 40.8 | |
| 130 | 3 | 4.4 | 12.7 | 20.5 | 26.2 | 30.2 |
| 7 | 9.2 | 15.8 | 22.1 | 27.1 | 30.7 | |
| 15 | 21.2 | 23.9 | 27.1 | 30.1 | 32.6 | |
| 140 | 3 | 0.1 | 1.7 | 6.3 | 11.8 | 16.8 |
| 7 | 0.7 | 3.1 | 7.7 | 12.8 | 17.5 | |
| 15 | 6.9 | 9.4 | 12.9 | 16.6 | 20.2 | |
| 150 | 3 | 0.0 | 0.1 | 1.6 | 4.9 | 8.9 |
| 7 | 0.0 | 0.5 | 2.3 | 5.6 | 9.5 | |
| 15 | 1.9 | 3.2 | 5.6 | 8.7 | 12.1 | |
| Between lot & between lab variability CV = 7% | ||||||
|---|---|---|---|---|---|---|
| True mean (% of label) |
Method variability RSDr (%) |
Variability of nutrients within lot (CV of distribution as % of true mean) | ||||
| 10% | 20% | 30% | 40% | 50% | ||
| 125 | 3 | 30.3 | 33.3 | 36.1 | 38.4 | 40.2 |
| 7 | 32.1 | 34.4 | 36.8 | 38.8 | 40.4 | |
| 15 | 36.4 | 37.5 | 38.8 | 40.2 | 41.3 | |
| 130 | 3 | 16.1 | 20.3 | 24.7 | 28.6 | 31.7 |
| 7 | 18.5 | 21.9 | 25.8 | 29.3 | 32.1 | |
| 15 | 25.2 | 27.0 | 29.3 | 31.6 | 33.7 | |
| 140 | 3 | 3.3 | 6.1 | 10.2 | 14.7 | 18.8 |
| 7 | 4.8 | 7.5 | 11.4 | 15.5 | 19.4 | |
| 15 | 10.7 | 12.7 | 15.6 | 18.7 | 21.7 | |
| 150 | 3 | 0.5 | 1.5 | 3.8 | 7.1 | 10.8 |
| 7 | 1.0 | 2.2 | 4.6 | 7.8 | 11.3 | |
| 15 | 4.1 | 5.5 | 7.8 | 10.6 | 13.6 | |
Graphs 1.1 - 6.2 visually depict data provided in tables 1 - 3 to facilitate comparisons between different mean nutrient contents and different sources of variation.
Comparison of scenarios
Class I: Added vitamins and mineral nutrients,
Producer's risk (Type I error)
(Rejection level <100%)
Graph 1.1
True mean=110% of label
Between lot & between lab variability CV= 3%

Graph 1.2
True mean = 120% of label
Between lot & between lab variability CV = 3%

Class I: Vitamins and mineral nutrients,
Producer's risk (Type I error)
(Rejection level < 100%)
Graph 2.1
Within lab method variability RSDr = 7%
Between lot & between lab variability CV = 3%

Graph 2.2
Within lab method variability RSDr = 15%
Between lot & between lab variability CV = 3%

Class I: Added vitamins and mineral nutrients,
Consumer's risk (Type II error)
(Acceptance level ≥ 100%)
Graph 3.1
True mean = 90% of label
Between lot & between lab variability CV = 3%

Graph 3.2
True mean = 80% of label
Between lot & between lab variability CV = 3%

Class I: Added vitamins and mineral nutrients,
Consumer's risk (Type II error)
(Acceptance level ≥ 100%)
Graph 4.1
Within lab method variability RSDr = 7%
Between lot & between lab variability CV = 3%

Graph 4.2
Within lab method variability RSDr = 15%
Between lot & between lab variability CV = 3%

Class II: Calories, fat, saturated fat, trans fat, cholesterol, sodium, sugars
Producer's risk (Type I error)
(Rejection level > 120%)
Graph 5.1
True mean = 100% of label
Between lot & between lab variability CV = 3%

Graph 5.2
Within lab method variability RSDr = 7%
Between lot & between lab variability CV = 3%

Class II: Calories, fat, saturated fat, trans fat, cholesterol, sodium, sugars
Consumer's risk (Type II error)
(Acceptance level ≤ 120%)
Graph 6.1
True mean = 140% of label
Between lot & between lab variability CV = 3%

Graph 6.2
Within lab method variability RSDr = 7%
Between lot & between lab variability CV = 3%

B. Acceptance criteria for lot compliance
- The nutrient values are assumed to be normally distributed in the lot about the declared/label value. The consumer's risk and the producer's risk are evaluated based on this assumption which should hold for a stable production process. Under this assumption, since a nutrient value must be non-negative, the coefficient of variation (CV) should have an upper bound of 50%. This translates to the probability of any random sample taken from the lot giving a nutrient value less than half the declared value to be less than 0.16, and likewise for being greater than 1.5 the declared value. In other words, fewer than one in six such random samples selected from the lot should provide results that are not within 50% of the declared value. Consequently, if one of our three lab results is not within 50% of the label value, the validity of our assumption of an underlying normal distribution for the nutrient content is highly questionable. Having only three observations for our lot, standard statistical methods for testing normality are not applicable but this criterion should serve a similar purpose.
- This criterion serves as the nucleus of the compliance test by requiring the mean of the three lab results to fall within the allowable limit which is the declared value subjected to the appropriate rounding rules (Appendix 3) and subsequent applicable tolerance.
- With the growing fortification of foods, there may be safety issues associated with large variability. It would seem reasonable that where a nutrient has been added to a food an overall variability remain within 10% (0.1) of the mean value. This variability then should lie within the limits of a confidence interval for the standard deviation of the lot nutrient content. Recognizing that the variability within a lot as well as that between lots may vary significantly according to the nutrient under consideration, a 99% confidence level is preferred to the more commonly used 95% level of confidence. This allows greater elasticity in the estimate and is less stringent by providing a wider confidence interval. The above is equivalent to having 0.1 being greater than the 99% lower confidence limit, evaluated from the lab results as (s x 0.4344/
 ) where
) where  and s are respectively, the declared mean and the standard deviation of the three lab determinations for the lot nutrient content.
and s are respectively, the declared mean and the standard deviation of the three lab determinations for the lot nutrient content.
C. Glossary
- Accuracy:
- The closeness of agreement between a test result and the accepted reference value.
- Coefficient of variation (CV):
- This quantity expresses the standard deviation as a percentage of the mean. The coefficient of variation of a random variable X having a mean µ and variance
 is given as
is given as 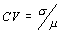
- Label value (or declared value):
- The amount of nutrient declared on the label or in advertising.
- Nutrition labelling compliance test:
- A test conducted by CFIA for verifying the accuracy of nutrient values on labels and in advertising. This test is based on a collection of units of product from which a sample is drawn and inspected to determine conformity with the acceptability criteria.
- Lot (or batch):
- A collection of identically labelled products produced under conditions as nearly uniform as possible and available for inspection at one time.
- Sample:
- A subset of units of product drawn from a lot or batch, that is representative of the lot for inspection purposes.
- Composite sub-sample:
- A subset of the sample units that are combined and mixed to homogeneity.
- Repeatability variance (R):
- The variance of independent test results obtained with the same method on identical test items in the same laboratory by the same operator using the same equipment within short intervals of time (RSDr)
- Reproducibility variance (B):
- The variance of test results obtained with the same method on identical test items in different laboratories with different operators using different equipment.
- Producer's risk (Type I error):
- The probability that a lot meeting the label claim will be deemed unacceptable.
- Consumer's risk (Type II error):
- The probability that a lot of unacceptable declared values is erroneously accepted as satisfactory
Appendix 3 - Rounding of nutrition facts table information
Table 1 summarizes the rounding rules for nutrition labelling information, consistent with section B.01.401 and B.01.402 of the Food and Drug Regulations (FDR). The table shows how a range of nutrient values is rounded off to a single value in the Nutrition Facts table. As the nutrient concentration increases, the range of values represented by the single rounded value also increases.
Table 2 shows the rounding and compliance limit impact for declarations of added vitamins and mineral nutrients where there is no tolerance.
Table 3 shows the impact of applying the tolerance to the minimum or maximum limit prior to rounding. Thus, for a label value listed in column A, the outer limits of the pre-rounding range that is represented by the label value are provided in columns B and C. Column D provides the tolerance amount (calculated as 20% of the declared amount) and Column E provides their minimum or maximum compliance limit of the nutrient amount that may be found in the sample with respect to a given label value (column A), for the lot to be accepted.
Example: Table 3, Class II, Fat - A value of 2.5 g per serving is declared: rounding is to the nearest 0.5 g, so the maximum limit prior to rounding (pre-round) is 2.74 g ; a further 20% tolerance or 0.5 g is added to the pre-round to give a maximum acceptable limit = 2.74+ 0.5 = 3.24 g. Therefore, a sample containing 3.24 g fat or less would be accepted for a product declaring 2.5 g fat per serving in the Nutrition Facts table.
Table 1 - Rounded values
| Nutrient | Condition | Rounding absolute amount |
Rounding % daily value |
|---|---|---|---|
| Energy (calories) | < 5 calories, meets "free of energy" | 0 calories | |
| Energy (calories) | < 5 calories, all other cases | nearest multiple of 1 Cal | |
| Energy (calories) | ≥ 5 to ≤ 50 calories | nearest multiple of 5 Cal | |
| Energy (calories) | > 50 calories | nearest multiple of 10 Cal | |
| Fat | < 0.5 g of fat, meets "free of fat", saturated fat and trans fat declared as "0 g" | 0 g | 0 % |
| Fat | < 0.5 g of fat, all other cases | nearest multiple of 0.1 g | nearest multiple of 1 % |
| Fat | ≥ 0.5 g to ≤ 5 g of fat | nearest multiple of 0.5 g | nearest multiple of 1 % |
| Fat | > 5 g of fat | nearest multiple of 1 g | nearest multiple of 1 % |
| Saturated fatty acids | < 0.5 g of saturated fat and meets "free of saturated fat" [< 0.2 g ] | 0 g | |
| Saturated fatty acids | < 0.5 g of saturated fat, all other cases | nearest multiple of 0.1 g | |
| Saturated fatty acids | ≥ 0.5 g to ≤ 5 g saturated fat | nearest multiple of 0.5 g | |
| Saturated fatty acids | > 5 g of saturated fat | nearest multiple of 1 g | |
| Trans fatty acids | < 0.5 g of trans fat and meets "free of trans fat" [< 0.2 g ] | 0 g | |
| Trans fatty acids | < 0.5 g of trans fat, all other cases | nearest multiple of 0.1 g | |
| Trans fatty acids | ≥ 0.5 g to ≤ 5 g of trans fat | nearest multiple of 0.5 g | |
| Trans fatty acids | > 5 g of trans fat | nearest multiple of 1 g | |
| Sum of saturated fatty acids and trans fatty acids | Saturated fat and trans fat declared as "0 g" | 0 % | |
| Sum of saturated fatty acids and trans fatty acids | all other cases | nearest multiple of 1 % | |
| Polyunsaturated fatty acids Omega-6 polyunsaturated fatty acids Omega-3 polyunsaturated fatty acids Monounsaturated fatty acids |
< 1 g | nearest multiple of 0.1 g | |
| Polyunsaturated fatty acids Omega-6 polyunsaturated fatty acids Omega-3 polyunsaturated fatty acids Monounsaturated fatty acids |
≥ 1 g to ≤ 5 g | nearest multiple of 0.5 g | |
| Polyunsaturated fatty acids Omega-6 polyunsaturated fatty acids Omega-3 polyunsaturated fatty acids Monounsaturated fatty acids |
> 5 g | nearest multiple of 1 g | |
| Carbohydrate | < 0.5 g | 0 g | |
| Carbohydrate | ≥ 0.5 g | nearest multiple of 1 g | |
| Fibre | < 0.5 g | 0 g | 0 % |
| Fibre | ≥ 0.5 g | nearest multiple of 1 g | nearest multiple of 1 % |
| Sugars | < 0.5 g | 0 g | 0 % |
| Sugars | ≥ 0.5 g | nearest multiple of 1 g | nearest multiple of 1 % |
| Protein | < 0.5 g | nearest multiple of 0.1 g | |
| Protein | ≥ 0.5 g | nearest multiple of 1 g | |
| Cholesterol | < 2 mg and meets "free of cholesterol" | 0 mg | 0 % (optional) |
| Cholesterol | all other cases | nearest multiple of 5 mg | nearest multiple of 1 % (optional) |
| Sodium | < 5 mg and meets "free of sodium or salt" | 0 mg | 0 % |
| Sodium | < 5 mg, all other cases | nearest multiple of 1 mg | nearest multiple of 1 % |
| Sodium | ≥ 5 mg to ≤ 140 mg | nearest multiple of 5 mg | nearest multiple of 1 % |
| Sodium | > 140 mg | nearest multiple of 10 mg | nearest multiple of 1 % |
| Potassium Calcium Phosphorous |
< 5 mg | 0 mg | 0 % |
| Potassium Calcium Phosphorous |
≥ 5 mg to < 50 mg | nearest multiple of 10 mg | nearest multiple of 1 % |
| Potassium Calcium Phosphorous |
≥50 mg to < 250 mg | nearest multiple of 25 mg | nearest multiple of 1 % |
| Potassium Calcium Phosphorous |
≥ 250 mg | nearest multiple of 50 mg | nearest multiple of 1 % |
| Iron Zinc Vitamin E |
< 0.05 mg | 0 mg | 0 % |
| Iron Zinc Vitamin E |
≥ 0.05 mg to < 0.5 mg | nearest multiple of 0.1 mg | nearest multiple of 1 % |
| Iron Zinc Vitamin E |
≥ 0.5 mg to < 2.5 mg | nearest multiple of 0.25 mg | nearest multiple of 1 % |
| Iron Zinc Vitamin E |
≥ 2.5 mg | nearest multiple of 0.5 mg | nearest multiple of 1 % |
| Vitamin A | < 5 µg | 0 µg | 0 % |
| Vitamin A | ≥ 5 µg to < 50 µg | nearest multiple of 10 µg | nearest multiple of 1 % |
| Vitamin A | ≥ 50 µg to < 250 µg | nearest multiple of 50 µg | nearest multiple of 1 % |
| Vitamin A | ≥ 250 µg | nearest multiple of 100 µg | nearest multiple of 1 % |
| Vitamin C | < 0.1 mg | 0 mg | 0 % |
| Vitamin C | ≥ 0.1 mg to < 1 mg | nearest multiple of 0.2 mg | nearest multiple of 1 % |
| Vitamin C | ≥ 1 mg to < 5 mg | nearest multiple of 0.5 mg | nearest multiple of 1 % |
| Vitamin C | ≥ 5 mg | nearest multiple of 1 mg | nearest multiple of 1 % |
| Vitamin D | < 0.1 µg | 0 µg | 0 % |
| Vitamin D | ≥ 0.1µg to < 1 µg | nearest multiple of 0.2 µg | nearest multiple of 1 % |
| Vitamin D | ≥ 1 µg to < 5 µg | nearest multiple of 0.5 µg | nearest multiple of 1 % |
| Vitamin D | ≥ 5 µg | nearest multiple of 1 µg | nearest multiple of 1 % |
| Thiamin Riboflavin Manganese |
< 0.005 mg | 0 mg | 0 % |
| Thiamin Riboflavin Manganese |
≥ 0.005 mg to < 0.05 mg | nearest multiple of 0.01 mg | nearest multiple of 1 % |
| Thiamin Riboflavin Manganese |
≥ 0.05 mg to < 0.25 mg | nearest multiple of 0.025 mg | nearest multiple of 1 % |
| Thiamin Riboflavin Manganese |
≥ 0.25 mg | nearest multiple of 0.05 mg | nearest multiple of 1 % |
| Magnesium | < 1 mg | 0 mg | 0 % |
| Magnesium | ≥ 1 mg to < 10 mg | nearest multiple of 2 mg | nearest multiple of 1 % |
| Magnesium | ≥ 10 mg to < 50 mg | nearest multiple of 5 mg | nearest multiple of 1 % |
| Magnesium | ≥ 50 mg | nearest multiple of 10 mg | nearest multiple of 1 % |
| Copper | < 0.0015 mg | 0 mg | 0 % |
| Copper | ≥ 0.0015 mg to < 0.025 mg | nearest multiple of 0.002 mg | nearest multiple of 1 % |
| Copper | ≥ 0.025 mg to < 0.05 mg | nearest multiple of 0.005 mg | nearest multiple of 1 % |
| Copper | ≥ 0.05 mg | nearest multiple of 0.01 mg | nearest multiple of 1 % |
Table 2 - Rounding and compliance limit for class I - Added vitamins and mineral nutrients (absolute amount)
The mean nutrient content is not less than the declared (label) value adjusted for rounding.
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 10 | 5 | 5 |
| 20 | 15 | 15 |
| 30 | 25 | 25 |
| 40 | 35 | 35 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 50 | 37.5 | 37.5 |
| 75 | 62.5 | 62.5 |
| 100 | 87.5 | 87.5 |
| 125 | 112.5 | 112.5 |
| 150 | 137.5 | 137.5 |
| 175 | 162.5 | 162.5 |
| 200 | 187.5 | 187.5 |
| 225 | 212.5 | 212.5 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 250 | 225 | 225 |
| 300 | 275 | 275 |
| 350 | 325 | 325 |
| 400 | 375 | 375 |
| 450 | 425 | 425 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.1 | 0.05 | 0.05 |
| 0.2 | 0.15 | 0.15 |
| 0.3 | 0.25 | 0.25 |
| 0.4 | 0.35 | 0.35 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.50 | 0.375 | 0.375 |
| 0.75 | 0.625 | 0.625 |
| 1.00 | 0.875 | 0.875 |
| 1.25 | 1.125 | 1.125 |
| 1.50 | 1.375 | 1.375 |
| 1.75 | 1.625 | 1.625 |
| 2.00 | 1.875 | 1.875 |
| 2.25 | 2.125 | 2.125 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 2.5 | 2.25 | 2.25 |
| 3.0 | 2.75 | 2.75 |
| 3.5 | 3.25 | 3.25 |
| 4.0 | 3.75 | 3.75 |
| 4.5 | 4.25 | 4.25 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 10 | 5 | 5 |
| 20 | 15 | 15 |
| 30 | 25 | 25 |
| 40 | 35 | 35 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 50 | 45 | 45 |
| 100 | 75 | 75 |
| 150 | 125 | 125 |
| 200 | 175 | 175 |
| 250 | 225 | 225 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 300 | 250 | 250 |
| 400 | 350 | 350 |
| 500 | 450 | 450 |
| 600 | 550 | 550 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.2 | 0.1 | 0.1 |
| 0.4 | 0.3 | 0.3 |
| 0.6 | 0.5 | 0.5 |
| 0.8 | 0.7 | 0.7 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 1.0 | 0.75 | 0.75 |
| 1.5 | 1.25 | 1.25 |
| 2.0 | 1.75 | 1.75 |
| 2.5 | 2.25 | 2.25 |
| 3.0 | 2.75 | 2.75 |
| 3.5 | 3.25 | 3.25 |
| 4.0 | 3.75 | 3.75 |
| 4.5 | 4.25 | 4.25 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 5 | 4.5 | 4.5 |
| 6 | 5.5 | 5.5 |
| 7 | 6.5 | 6.5 |
| 8 | 7.5 | 7.5 |
| 9 | 8.5 | 8.5 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.2 | 0.1 | 0.1 |
| 0.4 | 0.3 | 0.3 |
| 0.6 | 0.5 | 0.5 |
| 0.8 | 0.7 | 0.7 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 1.0 | 0.75 | 0.75 |
| 1.5 | 1.25 | 1.25 |
| 2.0 | 1.75 | 1.75 |
| 2.5 | 2.25 | 2.25 |
| 3.0 | 2.75 | 2.75 |
| 3.5 | 3.25 | 3.35 |
| 4.0 | 3.75 | 3.75 |
| 4.5 | 4.25 | 4.25 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 5 | 4.5 | 4.5 |
| 6 | 5.5 | 5.5 |
| 7 | 6.5 | 6.5 |
| 8 | 7.5 | 7.5 |
| 9 | 8.5 | 8.5 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.01 | 0.005 | 0.005 |
| 0.02 | 0.015 | 0.015 |
| 0.03 | 0.025 | 0.025 |
| 0.04 | 0.035 | 0.035 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.050 | 0.0375 | 0.0375 |
| 0.075 | 0.0625 | 0.0625 |
| 0.100 | 0.0875 | 0.0875 |
| 0.125 | 0.1125 | 0.1125 |
| 0.150 | 0.1375 | 0.1375 |
| 0.175 | 0.1625 | 0.1625 |
| 0.200 | 0.1875 | 0.1875 |
| 0.225 | 0.2125 | 0.2125 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.25 | 0.225 | 0.225 |
| 0.30 | 0.275 | 0.275 |
| 0.35 | 0.325 | 0.325 |
| 0.40 | 0.375 | 0.375 |
| 0.45 | 0.425 | 0.425 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 2 | 1 | 1 |
| 4 | 3 | 3 |
| 6 | 5 | 5 |
| 8 | 7 | 7 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 10 | 7.5 | 7.5 |
| 15 | 12.5 | 12.5 |
| 20 | 17.5 | 17.5 |
| 25 | 22.5 | 22.5 |
| 30 | 27.5 | 27.5 |
| 35 | 32.5 | 32.5 |
| 40 | 37.5 | 37.5 |
| 45 | 42.5 | 42.5 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 50 | 45 | 45 |
| 60 | 55 | 55 |
| 70 | 65 | 65 |
| 80 | 75 | 75 |
| 90 | 85 | 85 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.002 | 0.001 | 0.001 |
| 0.004 | 0.003 | 0.003 |
| 0.006 | 0.005 | 0.005 |
| 0.008 | 0.007 | 0.007 |
| 0.010 | 0.009 | 0.009 |
| 0.012 | 0.011 | 0.011 |
| 0.014 | 0.013 | 0.013 |
| 0.016 | 0.015 | 0.015 |
| 0.018 | 0.017 | 0.017 |
| 0.020 | 0.019 | 0.019 |
| 0.022 | 0.021 | 0.021 |
| 0.024 | 0.023 | 0.023 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.025 | 0.0225 | 0.0225 |
| 0.030 | 0.0275 | 0.0250 |
| 0.035 | 0.0325 | 0.0325 |
| 0.040 | 0.0375 | 0.0375 |
| 0.045 | 0.0425 | 0.0425 |
| A label value |
B minimum pre-round |
C compliance limit |
|---|---|---|
| 0.05 | 0.045 | 0.045 |
| 0.06 | 0.055 | 0.055 |
| 0.07 | 0.065 | 0.065 |
| 0.08 | 0.075 | 0.075 |
| 0.09 | 0.085 | 0.085 |
Table 3 - Rounding and compliance limit for class II (absolute amount)
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 0 | 0 | 4.99 | 1.0 | 6.0 Table note o |
| 1 | 0.5 | 1.4 | 0.2 | 1.6 |
| 2 | 1.5 | 2.4 | 0.4 | 2.8 |
| 3 | 2.5 | 3.4 | 0.6 | 4.0 |
| 4 | 3.5 | 4.4 | 0.8 | 5.2 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 5 | 4.5 | 7.4 | 1.0 | 8.4 |
| 10 | 7.5 | 12.4 | 2.0 | 14.4 |
| 15 | 12.5 | 17.4 | 3.0 | 20.4 |
| 20 | 17.5 | 22.4 | 4.0 | 26.4 |
| 25 | 22.5 | 27.4 | 5.0 | 32.4 |
| 30 | 27.5 | 32.4 | 6.0 | 38.4 |
| 35 | 32.5 | 37.4 | 7.0 | 44.4 |
| 40 | 37.5 | 42.4 | 8.0 | 50.4 |
| 45 | 42.5 | 47.4 | 9.0 | 56.4 |
| 50 | 47.5 | 52.4 | 10.0 | 62.4 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 60 | 55 | 64 | 12 | 76 |
| 70 | 65 | 74 | 14 | 88 |
| 80 | 75 | 84 | 16 | 100 |
| 90 | 85 | 94 | 18 | 112 |
| 100 | 95 | 104 | 20 | 124 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 0 | 0 | 0.199 | 0.04 | 0.24 Table note p |
| 0 | 0 | 0.499 | 0.10 | 0.60 Table note q |
| 0.1 | 0.05 | 0.14 | 0.02 | 0.16 |
| 0.2 | 0.15 | 0.24 | 0.04 | 0.28 |
| 0.3 | 0.25 | 0.34 | 0.06 | 0.40 |
| 0.4 | 0.35 | 0.44 | 0.08 | 0.52 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 0.5 | 0.45 | 0.74 | 0.10 | 0.84 |
| 1.0 | 0.75 | 1.24 | 0.20 | 1.44 |
| 1.5 | 1.25 | 1.74 | 0.30 | 2.04 |
| 2.0 | 1.75 | 2.24 | 0.40 | 2.64 |
| 2.5 | 2.25 | 2.74 | 0.50 | 3.24 |
| 3.0 | 2.75 | 3.24 | 0.60 | 3.84 |
| 3.5 | 3.25 | 3.74 | 0.70 | 4.44 |
| 4.0 | 3.75 | 4.24 | 0.80 | 5.04 |
| 4.5 | 4.25 | 4.74 | 0.90 | 5.64 |
| 5.0 | 4.75 | 5.24 | 1.00 | 6.24 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 6 | 5.5 | 6.4 | 1.2 | 7.6 |
| 7 | 6.5 | 7.4 | 1.4 | 8.8 |
| 8 | 7.5 | 8.4 | 1.6 | 10.0 |
| 9 | 8.5 | 9.4 | 1.8 | 11.2 |
| 10 | 9.5 | 10.4 | 2.0 | 12.4 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.1 | 0.05 | 0.14 | 0.02 | 0.03 |
| 0.2 | 0.15 | 0.24 | 0.04 | 0.11 |
| 0.3 | 0.25 | 0.34 | 0.06 | 0.19 |
| 0.4 | 0.35 | 0.44 | 0.08 | 0.27 |
| 0.5 | 0.45 | 0.54 | 0.10 | 0.35 |
| 0.6 | 0.55 | 0.64 | 0.12 | 0.43 |
| 0.7 | 0.65 | 0.74 | 0.14 | 0.51 |
| 0.8 | 0.75 | 0.84 | 0.16 | 0.59 |
| 0.9 | 0.85 | 0.94 | 0.18 | 0.67 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 1.0 | 0.95 | 1.24 | 0.20 | 0.75 |
| 1.5 | 1.25 | 1.74 | 0.30 | 0.95 |
| 2.0 | 1.75 | 2.24 | 0.40 | 1.35 |
| 2.5 | 2.25 | 2.74 | 0.50 | 1.75 |
| 3.0 | 2.75 | 3.24 | 0.60 | 2.15 |
| 3.5 | 3.25 | 3.74 | 0.70 | 2.55 |
| 4.0 | 3.75 | 4.24 | 0.80 | 2.95 |
| 4.5 | 4.25 | 4.74 | 0.90 | 3.35 |
| 5.0 | 4.75 | 5.24 | 1.00 | 3.75 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 6 | 5.5 | 6.4 | 1.2 | 4.3 |
| 7 | 6.5 | 7.4 | 1.4 | 5.1 |
| 8 | 7.5 | 8.4 | 1.6 | 5.9 |
| 9 | 8.5 | 9.4 | 1.8 | 6.7 |
| 10 | 9.5 | 10.4 | 2.0 | 7.5 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre-round - 20% of label) |
|---|---|---|---|---|
| 1 | 0.5 | 1.4 | 0.2 | 0.3 |
| 2 | 1.5 | 2.4 | 0.4 | 1.1 |
| 3 | 2.5 | 3.4 | 0.6 | 1.9 |
| 4 | 3.5 | 4.4 | 0.8 | 2.7 |
| 5 | 4.5 | 5.4 | 1.0 | 3.5 |
| 6 | 5.5 | 6.4 | 1.2 | 4.3 |
| 7 | 6.5 | 7.4 | 1.4 | 5.1 |
| 8 | 7.5 | 8.4 | 1.6 | 5.9 |
| 9 | 8.5 | 9.4 | 1.8 | 6.7 |
| 10 | 9.5 | 10.4 | 2.0 | 7.5 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 0 | 0 | 0.499 | 0.10 | 0.60 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre-round + 20% of label) |
|---|---|---|---|---|
| 1 | 0.5 | 1.4 | 0.2 | 1.6 |
| 2 | 1.5 | 2.4 | 0.4 | 2.8 |
| 3 | 2.5 | 3.4 | 0.6 | 4.0 |
| 4 | 3.5 | 4.4 | 0.8 | 5.2 |
| 5 | 4.5 | 5.4 | 1.0 | 6.4 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.1 | 0.05 | 0.14 | 0.02 | 0.03 |
| 0.2 | 0.15 | 0.24 | 0.04 | 0.11 |
| 0.3 | 0.25 | 0.34 | 0.06 | 0.19 |
| 0.4 | 0.35 | 0.44 | 0.08 | 0.27 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 1 | 0.5 | 1.4 | 0.2 | 0.3 |
| 2 | 1.5 | 2.4 | 0.4 | 1.1 |
| 3 | 2.5 | 3.4 | 0.6 | 1.9 |
| 4 | 3.5 | 4.4 | 0.8 | 2.7 |
| 5 | 4.5 | 5.4 | 1.0 | 3.5 |
| 6 | 5.5 | 6.4 | 1.2 | 4.3 |
| 7 | 6.5 | 7.4 | 1.4 | 5.1 |
| 8 | 7.5 | 8.4 | 1.6 | 5.9 |
| 9 | 8.5 | 9.4 | 1.8 | 6.7 |
| 10 | 9.5 | 10.4 | 2.0 | 7.5 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 0 | 0 | 1.99 | 0.4 | 2.4 Table note r |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 5 | 2.5 | 7.4 | 1.0 | 8.4 |
| 10 | 7.5 | 12.4 | 2.0 | 14.4 |
| 15 | 12.5 | 17.4 | 3.0 | 20.4 |
| 20 | 17.5 | 22.4 | 4.0 | 26.4 |
| 25 | 22.5 | 27.4 | 5.0 | 32.4 |
| 30 | 27.5 | 32.4 | 6.0 | 38.4 |
| 50 | 47.5 | 52.4 | 10.0 | 62.4 |
| 100 | 97.5 | 102.4 | 20.0 | 122.4 |
| 200 | 197.5 | 202.4 | 40.0 | 242.4 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 0 | 0 | 4.99 | 1.0 | 6.0 Table note s |
| 1 | 0.5 | 1.4 | 0.2 | 1.6 |
| 2 | 1.5 | 2.4 | 0.4 | 2.8 |
| 3 | 2.5 | 3.4 | 0.6 | 4.0 |
| 4 | 3.5 | 4.4 | 0.8 | 5.2 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 5 | 4.5 | 7.4 | 1.0 | 8.4 |
| 10 | 7.5 | 12.4 | 2.0 | 14.4 |
| 15 | 12.5 | 17.4 | 3.0 | 20.4 |
| 20 | 17.5 | 22.4 | 4.0 | 26.4 |
| 25 | 22.5 | 27.4 | 5.0 | 32.4 |
| 30 | 27.5 | 32.4 | 6.0 | 38.4 |
| 35 | 32.5 | 37.4 | 7.0 | 44.4 |
| 40 | 37.5 | 42.4 | 8.0 | 50.4 |
| 45 | 42.5 | 47.4 | 9.0 | 56.4 |
| 50 | 47.5 | 52.4 | 10.0 | 62.4 |
| 55 | 52.5 | 57.4 | 11.0 | 68.4 |
| 60 | 57.5 | 62.4 | 12.0 | 74.4 |
| 65 | 62.5 | 67.4 | 13.0 | 80.4 |
| 70 | 67.5 | 72.4 | 14.0 | 86.4 |
| 75 | 72.5 | 77.4 | 15.0 | 92.4 |
| 80 | 77.5 | 82.4 | 16.0 | 98.4 |
| 85 | 82.5 | 87.4 | 17.0 | 104.4 |
| 90 | 87.5 | 92.4 | 18.0 | 110.4 |
| 95 | 92.5 | 97.4 | 19.0 | 116.4 |
| 100 | 97.5 | 102.4 | 20.0 | 122.4 |
| 105 | 102.5 | 107.4 | 21.0 | 128.4 |
| 110 | 107.5 | 112.4 | 22.0 | 134.4 |
| 115 | 112.5 | 117.4 | 23.0 | 140.4 |
| 120 | 117.5 | 122.4 | 24.0 | 146.4 |
| 125 | 122.5 | 127.4 | 25.0 | 152.4 |
| 130 | 127.5 | 132.4 | 26.0 | 158.4 |
| 135 | 132.5 | 137.4 | 27.0 | 164.4 |
| 140 | 137.5 | 142.4 | 28.0 | 170.4 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (C+D) compliance limit = (maximum pre- round + 20% of label) |
|---|---|---|---|---|
| 150 | 145 | 154 | 30 | 184 |
| 200 | 195 | 204 | 40 | 244 |
| 300 | 295 | 304 | 60 | 364 |
| 400 | 395 | 404 | 80 | 484 |
| 500 | 495 | 504 | 100 | 604 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 10 | 5 | 14 | 2 | 3 |
| 20 | 15 | 24 | 4 | 11 |
| 30 | 25 | 34 | 6 | 19 |
| 40 | 35 | 44 | 8 | 27 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 50 | 37.5 | 62.4 | 10.0 | 27.5 |
| 75 | 62.5 | 87.4 | 15.0 | 47.5 |
| 100 | 87.5 | 112.4 | 20.0 | 67.5 |
| 125 | 112.5 | 137.4 | 25.0 | 87.5 |
| 150 | 137.5 | 162.4 | 30.0 | 107.5 |
| 175 | 162.5 | 187.4 | 35.0 | 127.5 |
| 200 | 187.5 | 212.4 | 40.0 | 147.5 |
| 225 | 212.5 | 237.4 | 45.0 | 167.5 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 250 | 225 | 274 | 50 | 175 |
| 300 | 275 | 324 | 60 | 215 |
| 350 | 325 | 374 | 70 | 255 |
| 400 | 375 | 424 | 80 | 295 |
| 450 | 425 | 474 | 90 | 335 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.1 | 0.05 | 0.14 | 0.02 | 0.03 |
| 0.2 | 0.15 | 0.24 | 0.04 | 0.11 |
| 0.3 | 0.25 | 0.34 | 0.06 | 0.19 |
| 0.4 | 0.35 | 0.44 | 0.08 | 0.27 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.50 | 0.375 | 0.624 | 0.100 | 0.275 |
| 0.75 | 0.625 | 0.874 | 0.150 | 0.475 |
| 1.00 | 0.875 | 1.124 | 0.200 | 0.675 |
| 1.25 | 1.125 | 1.374 | 0.250 | 0.875 |
| 1.50 | 1.375 | 1.624 | 0.300 | 1.075 |
| 1.75 | 1.625 | 1.874 | 0.350 | 1.275 |
| 2.00 | 1.875 | 2.124 | 0.400 | 1.475 |
| 2.25 | 2.125 | 2.374 | 0.450 | 1.675 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 2.5 | 2.25 | 2.74 | 0.50 | 1.75 |
| 3.0 | 2.75 | 3.24 | 0.60 | 2.15 |
| 3.5 | 3.25 | 3.74 | 0.70 | 2.55 |
| 4.0 | 3.75 | 4.24 | 0.80 | 2.95 |
| 4.5 | 4.25 | 4.74 | 0.90 | 3.35 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 10 | 5 | 14 | 2 | 3 |
| 20 | 15 | 24 | 4 | 11 |
| 30 | 25 | 34 | 6 | 19 |
| 40 | 35 | 44 | 8 | 27 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 50 | 45 | 74 | 10 | 35 |
| 100 | 75 | 124 | 20 | 55 |
| 150 | 125 | 174 | 30 | 95 |
| 200 | 175 | 224 | 40 | 135 |
| 250 | 225 | 274 | 50 | 175 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 300 | 250 | 349 | 60 | 190 |
| 400 | 350 | 449 | 80 | 270 |
| 500 | 450 | 549 | 100 | 350 |
| 600 | 550 | 649 | 120 | 430 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.2 | 0.10 | 0.29 | 0.04 | 0.06 |
| 0.4 | 0.30 | 0.49 | 0.08 | 0.22 |
| 0.6 | 0.50 | 0.69 | 0.12 | 0.38 |
| 0.8 | 0.70 | 0.89 | 0.16 | 0.54 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 1.0 | 0.75 | 1.24 | 0.20 | 0.55 |
| 1.5 | 1.25 | 1.74 | 0.30 | 0.95 |
| 2.0 | 1.75 | 2.24 | 0.40 | 1.35 |
| 2.5 | 2.25 | 2.74 | 0.50 | 1.75 |
| 3.0 | 2.75 | 3.24 | 0.60 | 2.15 |
| 3.5 | 3.25 | 3.74 | 0.70 | 2.55 |
| 4.0 | 3.75 | 4.24 | 0.80 | 2.95 |
| 4.5 | 4.25 | 4.74 | 0.90 | 3.35 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 5 | 4.5 | 5.4 | 1.0 | 3.5 |
| 6 | 5.5 | 6.4 | 1.2 | 4.3 |
| 7 | 6.5 | 7.4 | 1.4 | 5.1 |
| 8 | 7.5 | 8.4 | 1.6 | 5.9 |
| 9 | 8.5 | 9.4 | 1.8 | 6.7 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.2 | 0.10 | 0.29 | 0.04 | 0.06 |
| 0.4 | 0.30 | 0.49 | 0.08 | 0.22 |
| 0.6 | 0.50 | 0.69 | 0.12 | 0.38 |
| 0.8 | 0.70 | 0.89 | 0.16 | 0.54 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 1.0 | 0.75 | 1.24 | 0.20 | 0.55 |
| 1.5 | 1.25 | 1.74 | 0.30 | 0.95 |
| 2.0 | 1.75 | 2.24 | 0.40 | 1.35 |
| 2.5 | 2.25 | 2.74 | 0.50 | 1.75 |
| 3.0 | 2.75 | 3.24 | 0.60 | 2.15 |
| 3.5 | 3.25 | 3.74 | 0.70 | 2.55 |
| 4.0 | 3.75 | 4.24 | 0.80 | 2.95 |
| 4.5 | 4.25 | 4.74 | 0.90 | 3.35 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 5 | 4.5 | 5.4 | 1.0 | 3.5 |
| 6 | 5.5 | 6.4 | 1.2 | 4.3 |
| 7 | 6.5 | 7.4 | 1.4 | 5.1 |
| 8 | 7.5 | 8.4 | 1.6 | 5.9 |
| 9 | 8.5 | 9.4 | 1.8 | 6.7 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.01 | 0.005 | 0.014 | 0.002 | 0.003 |
| 0.02 | 0.015 | 0.024 | 0.004 | 0.011 |
| 0.03 | 0.025 | 0.034 | 0.006 | 0.019 |
| 0.04 | 0.035 | 0.044 | 0.008 | 0.027 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.050 | 0.0375 | 0.0624 | 0.0100 | 0.0275 |
| 0.075 | 0.0625 | 0.0874 | 0.0150 | 0.0475 |
| 0.100 | 0.0875 | 0.1124 | 0.0200 | 0.0675 |
| 0.125 | 0.1125 | 0.1374 | 0.0250 | 0.0875 |
| 0.150 | 0.1375 | 0.1624 | 0.0300 | 0.1075 |
| 0.175 | 0.1625 | 0.1874 | 0.0350 | 0.1275 |
| 0.200 | 0.1875 | 0.2124 | 0.0400 | 0.1475 |
| 0.225 | 0.2125 | 0.2374 | 0.0450 | 0.1675 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.25 | 0.225 | 0.274 | 0.050 | 0.175 |
| 0.30 | 0.275 | 0.324 | 0.060 | 0.215 |
| 0.35 | 0.325 | 0.374 | 0.070 | 0.255 |
| 0.40 | 0.375 | 0.424 | 0.080 | 0.295 |
| 0.45 | 0.425 | 0.474 | 0.090 | 0.335 |
| 0.50 | 0.475 | 0.524 | 0.100 | 0.375 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 2 | 1.0 | 2.9 | 0.4 | 0.6 |
| 4 | 3.0 | 4.9 | 0.8 | 2.2 |
| 6 | 5.0 | 6.9 | 1.2 | 3.8 |
| 8 | 7.0 | 8.9 | 1.6 | 5.4 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 10 | 7.5 | 12.4 | 2.0 | 5.5 |
| 15 | 12.5 | 17.4 | 3.0 | 9.5 |
| 20 | 17.5 | 22.4 | 4.0 | 13.5 |
| 25 | 22.5 | 27.4 | 5.0 | 17.5 |
| 30 | 27.5 | 32.4 | 6.0 | 21.5 |
| 35 | 32.5 | 37.4 | 7.0 | 25.5 |
| 40 | 37.5 | 42.4 | 8.0 | 29.5 |
| 45 | 42.5 | 47.4 | 9.0 | 33.5 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 50 | 45 | 54 | 10 | 35 |
| 60 | 55 | 64 | 12 | 43 |
| 70 | 65 | 74 | 14 | 51 |
| 80 | 75 | 84 | 16 | 59 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.002 | 0.0010 | 0.0029 | 0.0004 | 0.0006 |
| 0.004 | 0.0030 | 0.0049 | 0.0008 | 0.0022 |
| 0.006 | 0.0050 | 0.0069 | 0.0012 | 0.0038 |
| 0.008 | 0.0070 | 0.0089 | 0.0016 | 0.0054 |
| 0.010 | 0.0090 | 0.0109 | 0.0020 | 0.0070 |
| 0.012 | 0.0110 | 0.0129 | 0.0024 | 0.0086 |
| 0.016 | 0.0150 | 0.0169 | 0.0032 | 0.0118 |
| 0.018 | 0.0170 | 0.0189 | 0.0036 | 0.0134 |
| 0.020 | 0.0190 | 0.0209 | 0.0040 | 0.0150 |
| 0.022 | 0.0210 | 0.0229 | 0.0044 | 0.0166 |
| 0.024 | 0.0230 | 0.0249 | 0.0048 | 0.0182 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.025 | 0.0240 | 0.0274 | 0.0050 | 0.0190 |
| 0.030 | 0.0275 | 0.0324 | 0.0060 | 0.0215 |
| 0.035 | 0.0325 | 0.0374 | 0.0070 | 0.0255 |
| 0.040 | 0.0375 | 0.0424 | 0.0080 | 0.0295 |
| 0.045 | 0.0425 | 0.0474 | 0.0090 | 0.0335 |
| A label value |
B minimum pre-round |
C maximum pre-round |
D 20% of label |
E = (B-D) compliance limit = (minimum pre- round - 20% of label) |
|---|---|---|---|---|
| 0.05 | 0.045 | 0.054 | 0.010 | 0.035 |
| 0.06 | 0.055 | 0.064 | 0.012 | 0.043 |
| 0.07 | 0.065 | 0.074 | 0.014 | 0.051 |
| 0.08 | 0.075 | 0.084 | 0.016 | 0.059 |
| 0.09 | 0.085 | 0.094 | 0.018 | 0.067 |
Appendix 4 - Laboratory issues
Methods of analysis
CFIA conducts laboratory tests to verify the accuracy of nutrition information. Methods of analysis currently used by the CFIA appear in the table below. The CFIA does not require other laboratories to use these methods.
It is recommended that manufacturers rely on laboratory testing to verify their own label declarations. The methods of analysis recommended are those published in the most recent version of the Official methods of analysis of AOAC International wherever possible. Other collaboratively studied methods such as those published by the American Oil Chemists' Society, American Association of Cereal Chemists, ISO, etc. would also be considered appropriate. In house or journal methods with adequate method validation data are another possible option for method selection. Methods should be validated for the food matrix being analyzed.
Laboratory accreditation
Laboratories in Canada are accredited by the Standards Council of Canada (SCC) or the Canadian Association for Laboratory Accreditation (CALA) and not by CFIA. Accredited laboratories will have a list of methods as part of their scope of accreditation. These methods are those considered by SCC and CALA during the accreditation process. When choosing accredited laboratories, the tests provided should be contained in their scope of accreditation. Laboratories should also strive to subscribe to proficiency testing schemes for each method listed in their scope.
Choice of laboratory
CFIA recommends the selection of laboratories that are accredited to the ISO/IEC 17025 standard by the Standards Council of Canada (SCC) or the Canadian Association for Laboratory Accreditation (CALA). ISO/IEC 17025 accredited laboratories from other countries would also be accepted. Company quality assurance laboratories using validated methods can also be used.
A list of accredited laboratories can be accessed through the Standards Council of Canada (SCC) or the Canadian Association for Laboratory Accreditation (CALA) web sites.
Table: Methods of analysis used by CFIA - Nutrition Facts table information
| Nutrient Table note t | Analytical method Table note u | Technique |
|---|---|---|
| Calories | LCAQ-040: Determination of carbohydrates and calories content in foods by difference Reference: Atwater Factors |
Application of appropriate factors to fat, protein, fibre and carbohydrate (may be adjusted for sugar alcohols and polydextrose) |
| Fat | LCAQ-034: Determination of fatty acids (C4-C24) in foods by GC-FID Reference: AOAC 996.06Table note v |
Gas chromatography-flame ionization detector (GC-FID) |
Fatty acids:
|
LCAQ-034: Determination of fatty acids (C4-C24) in foods by GC-FID Reference: AOAC 996.06Table note v |
Gas chromatography-flame ionization detector (GC-FID) |
| Cholesterol | LCAQ-035: Determination of cholesterol in foods by GC-FID Reference: AOAC 994.10Table note v |
Gas chromatography-flame ionization detector (GC-FID) |
| Carbohydrate | LCAQ-040: Determination of carbohydrates and calories content in foods by difference Carbohydrate = Total solids − (ash + protein + fat) |
Carbohydrate by difference |
| Fibre | AOAC 2017.16Table note v: Total dietary fibre in foods and food ingredients rapid integrated enzymatic-gravimetric-high-pressure liquid chromatography method | Enzymatic – gravimetric / high performance liquid chromatography (HPLC) |
| Sugars | LCAQ-123: Determination of 12 sugars and sugar alcohols (fructose, glucose, lactose, galactose, maltose, sucrose, erythritol, xylitol, sorbitol, mannitol, mannose and isomaltose) in foods by HPLC-RID | HPLC with refractive index detector |
Protein |
LCAQ-098: Determination of protein in foods by combustion – Dumas principle Reference: ISO 14891 – Determination of nitrogen content using the Dumas principle |
Combustion instrument |
| LCAQ-109: Determination of fat, moisture and protein in cheese, and butter by near-infrared | Near-infrared spectroscopy | |
| Vitamin A | AOAC 2012.10Table note v: Simultaneous determination of vitamins E and A in infant formula and adult nutritionals normal-phase high-performance liquid chromatography | Normal phase HPLC-DAD |
LCAQ-002: Determination of vitamin A in foods by HPLC-UV-Visible Reference: AOAC 992.04Table note v |
Normal phase HPLC-DAD | |
LCAQ-097: HPLC determination of beta-carotene in foods by HPLC-UV-Visible Reference: AOAC 2005.7Table note v |
Reversed phase HPLC-DAD | |
LCAQ-110: Determination of vitamin A in milk by HPLC-UV-Visible Reference: AOAC 2002.06Table note v |
Normal phase HPLC-DAD | |
LCAQ-124: Determination of vitamin A in fortified plant-based beverages by HPLC-UV-Visible Reference: AOAC 2002.06Table note v |
Normal phase HPLC-DAD | |
| Vitamin C | AOAC 2012.22Table note v: Vitamin C in infant formula and adult/pediatric nutritional formula liquid chromatography with ultraviolet detection (LC-UV) | Reverse phase HPLC-DAD |
| Thiamin (vitamin B1) | Determination of thiamin (vitamin B1) in foods by spectrofluorimetry Reference 1: AOAC 986.27Table note v Reference 2: AOAC 942.23Table note v |
Fluorescence detection |
| Riboflavin (vitamin B2) | LCAQ-088: Determination of riboflavin (vitamin B2) in plant-based beverages and cheeses by HPLC-FLD Reference: Riboflavin (vitamin B2) in foods and vitamin preparations. Fluorometric method. AOAC International, Official methods of analysis of AOAC International, 17th edition. Chapter 45 section 45.1-08, Final action 1971. #970.65 |
Reverse phase HPLC-FLD |
| Vitamin D | LCAQ-112: Determination of vitamin D in foods by UHPLC-MS/MS Reference: AOAC 2011.11Table note v |
Reversed phase ultra-high performance liquid chromatography (UHPLC) with triple quadruple (QQQ) mass spectrometer (MS/MS) |
| Vitamin E | LCAQ-094: Determination of vitamin E (alpha-tocopherol) in foods by HPLC-FLD Reference: Determination of vitamins A (retinol) and E (alpha-tocopherol) in foods by liquid chromatography, Journal of AOAC International, 2002, Vol. 85, No. 2 |
Reversed phase HPLC with fluorescence detection |
| Iodine | AOAC 2012.15Table note v: Total iodine in infant formula and adult/pediatric nutritional formula inductively coupled plasma-mass spectrometry (ICP-MS) | Microwave digestion / inductively coupled plasma-mass spectrometry (ICP-MS) |
Mineral nutrients:
|
LCAQ-102: Determination of minerals (Na, Ca, K, Mg, P, Mn, Fe, Cu, Zn, Se and Mo) in food by ICP-MS Reference 1: Wu, S., Feng, X., and Wittmeir, A. Microwave digestion of plant and grain reference materials in nitric acid or a mixture of nitric acid and hydrogen peroxide for the determination of multi-elements by inductively coupled plasma mass spectrometry, Journal of Analytical Atomic Spectrometry, 1997, 12:797-806 Reference 2: Dolan, S.P. and Capar, Stephen G. Multi-element analysis of food by microwave digestion and inductively coupled plasma-atomic emission spectrometry, Journal of Food Composition and Analysis, 2002, 15:593-615 |
Microwave digestion / inductively coupled plasma-mass spectrometry (ICP-MS) |