On this page
- 1. Introduction
- 2. EID elements
- 3. Equine description terms
- 4. Examination/assessment of the EID
- 5. Equine Lot Program
- 6. List of veterinary drugs not permitted for use in equine slaughtered for food with Canadian brand name examples
- 7. List of "essential" veterinary drugs permitted in equine with a 180 day withdrawal period with Canadian brand name examples
- 8. List of veterinary drugs safe for use in equine intended for food production for which withdrawal periods have been determined with Canadian brand name examples
1. Introduction
It is mandatory in Canada, for all licence holders (operators) engaged in equine slaughter for edible purposes, for each establishment where the activity is conducted with inspection services provided by the Canadian Food Inspection Agency (CFIA), to have, from the person who owned or had the possession, care or control of the equine before its arrival at the establishment, a record that contains identity and a complete medical history for all equine (domestic and imported) presented for slaughter.
These Food Animal Information Document (FAID) records are referred to as equine information documents (EID) and provide the operator with the necessary level of confidence that identified potential hazards associated with live equine animals have been recorded, and to the extent possible, prevented or controlled at the farm level and during transportation. The EID allows CFIA staff to judge if the required information by section 165 of the Safe Food for Canadians Regulations (SFCR) has been provided and that the operator is taking the necessary measures to evaluate incoming equine according to the written specification contained in their Preventive Control Plan (PCP).
A completed individual EID contains a standardized description of the animal, as well as a comprehensive record of the equine's medical history for at least the preceding 180 days. The information template provided in section 2 should be used by equine owners as an aid to provide all the required information for individual equine animals to the operator or adapted for use by the equine industry as long as it can offer the same level of confidence.
The completed and signed EID must accompany the equine, at the time of ownership transfer, to the buyer of the animal intended to slaughter.
An owner of a group(s)/lot(s) of equine animals, assembled with the intention of utilizing them as food animals, may be eligible to present the animals to slaughter via a collective (lot) declaration. For details refer to section 5.
2. EID elements
The information provided below represents the core elements of the EID for individual equine (horses, donkeys, zebras and their crosses) presented for slaughter in Canada and can be adapted with all the elements described, for use by the equine industry as long as it can offer the same level of confidence.
The EID must contain both written and visual identification, as well as medical history and declaration (Part 3), bearing the original signature, by the owner of the equine and any person who had the possession, care or control of it immediately before its arrival at the slaughter establishment. A provision included in the EID (see Part 3) allows for the transfer of a complete medical history when 1 or more transient agents are involved.
Alternate options to filling out the written description and picture identification below are given at the end of Part 1.
Part 1: Written and visual identification
a) Written information
Primary location of the animal
![]()
![]()
(Land location or legal address or premise identification number)
Primary use(s) of the animal
Circle 1 or more of the following:
Recreation/companion animal/pleasure riding, breeding, ranch/farm work, public work, private industry work, performance/sport/show, racing, rodeo, urine production, food animals production. If other please specify:
If other please specify: ![]()
Sex: mare/filly, gelding, stallion/colt (circle 1)
Month and year of birth (if known) ![]()
Country of birth (if known) ![]()
Height in hands (1 hand = 4 inches) ![]()
Refer to the EID, section 3 for terms to be used for the following equine colour and marking identification section.
Body colour : ![]()
Markings:
- Head

- Body

- Limbs:
- right front

- right hind

- left front

- left hind

- right front
The following supplemental identification items may be completed if applicable.
List visible acquired marks (brands, tattoos, scars, etc.) and location
![]()
Pedigree registry and registration number
![]()
Microchip number and location
![]()
Passport ID number ![]()
Unique equine life number ![]()
Or other unique identifier ![]()
In the case of an EID completed by the previous owner, name of previous owner:
![]()
b) Picture identification
Attach 1 or more pages containing colour pictures of the animal showing the details contained in each of the views of the silhouettes below. The pictures must be clear and large enough to see the detail required. If applicable, take close ups of any visible acquired marks such as tattoos and attach. Owners ensure that the written description and pictures agree.
Silhouette
For information on how to complete the silhouette, refer to section 3.

Description for Silhouette:
5 silhouettes showing us: the right side view of a horse, the left side view of a horse, the face, the front view of the forelegs and the rear view of the hind legs.
Alternatives to written and picture identification
The following are alternative means of providing written and/or visual identification information. Note that the primary location of the animal and the primary use of the animal as indicated in Part 1a) is also required information.
- as a substitute for pictures, the above silhouette is completed, preferably by a licensed veterinarian or an authorized person Footnote 1
- ensure that the written description (Part 1a) and the completed silhouette agree
- official pedigree registration papers with written description and visual identification
- a copy of the registration papers is attached to the EID
- an official passport
- the passport is attached to the EID
- a complete EID (including Part 1) provided by a previous owner(s)
- the previous complete EID is attached to the current EID
- the name of the previous owner must appear in Part 1a) of the current EID
Part 2: Medical history
I, ![]() (name of owner) of
(name of owner) of ![]() (
(![]() )
) ![]() -
- ![]() (state your full contact address: street number or post office box number/city/province or state/postal code or zip code, phone number) am the ownerFootnote 2 of the animal identified on this document and have had uninterrupted possession, care or control of this animal from
(state your full contact address: street number or post office box number/city/province or state/postal code or zip code, phone number) am the ownerFootnote 2 of the animal identified on this document and have had uninterrupted possession, care or control of this animal from ![]() (indicate start date) to
(indicate start date) to ![]() (indicate end date).
(indicate end date).
Have any drugs or vaccines been administered to or consumed by the animal during the last 180 days?
Circle Yes or No
If yes, write the name the drug(s) or vaccine(s), Drug Identification Number (DIN) or an equivalent, if indicated on the label, last date of use, withdrawal period(s)Footnote 3, route of administration and for drugs, the amount used (dose) per treatment if the label does not indicate a dose or if drug is used at a dosage different than the label indicates.


Has the animal identified on this document shown signs of any illness or deviation from normal behaviour or appearance during the last 180 days?
Are there any physical, chemical hazards or other additional items to which the animal may have been exposed that could impact food safety that should be declared such as broken needles?
Circle Yes or No
If yes, provide details/descriptions.


-
Has the animal identified on this document been treated with a substance listed under the table named substances not permitted for use in food producing equine found in section 6?
Circle Yes or No
Part 3: Declarations
Owner declaration
As the owner of the animal identified on this document, I hereby certify that the information stated in this EID is accurate and complete.
I understand that at least 180 continuous days of documented history covering the time period before slaughter is required by the Safe Food for Canadians Regulations (SFCR) for an equine presented for processing in an establishment that is identified in the licence for the activities with inspection services provided by the Canadian Food Inspection Agency. As such, I have the option of attaching to this document, completed Equine Information Document(s) (EID(s)) from previous owner(s) in order to cover the required 180 continuous days of documented history.
![]()
(Signature of owner or the entrusted person)
![]() /
/![]() /
/![]()
(Date DD/MM/YY)
and clearly include the contact information of the entrusted person (that is, at least, name, title (for example: trainer), address and phone number)
![]()
![]()
(![]() )
) ![]() -
- ![]()
Transient agent declaration(s)
A transient agent is a person who maintains responsibility for the care of equine from the time of purchase for slaughter until their arrival at a meat processing establishment identified in a licence in Canada.
A transient agent declaration is applicable for an animal destined for slaughter shortly (the time needed to assemble, schedule, and move to slaughter) and may not be used in lieu of an ownership declaration. In the case of more than 1 transient agent caring for the animal(s) at different times, the transient agent declaration may be repeated on the EID as many times as necessary to cover the time period prior to slaughter.
Name of agent ![]()
Phone number (![]() )
) ![]() -
- ![]()
Address ![]()
The animal identified on this document has been under my care and control from ![]() (date) to
(date) to ![]() (date).
(date).
During this time period:
has this animal shown signs of any illness or deviation from normal behaviour or appearance?
Yes or No
If yes, provide details:


have any drugs or vaccines been administered to or consumed by the animal?
Yes or No
If yes, provide details:
Any drugs or vaccines administered to or consumed by the animal Drug or vaccine Drug Identification Number (DIN) or an equivalent Last date of use Withdrawal period Amount used (dose) per treatment Route of administration
Signature of agent ![]()
3. Equine written description terms
The EID requires an accurate standardized written description and visual identification that may include a completed equine outline instead of picture identification. The following terms must be used to complete the written description portion of the EID. In addition to brands or tattoos that the horse may bear, look for and identify unique distinguishing marks such as scars. Descriptive nomenclature for colouring and markings of equine as well as instructions for filling out the equine silhouette have been standardized by the International Equestrian Federation and have been adapted for use.
Height
The height of a horse is normally recorded in "hands", measured at the top of the withers.
"1 hand" equals 4 inches.
Colour
Appaloosa:
body colour is grey, covered with a mosaic of black or brown spots
Bay:
bay varies considerably in shade from dull red approaching brown, to a yellowish colour approaching chestnut, but it can be distinguished from the chestnut by the fact that the bay has a black mane and tail and almost invariably has black on the limbs and tips of the ears
Bay-brown:
the predominant colour is brown, with muzzle bay, black limbs, mane and tail
Black:
black pigment is general throughout the coat, limbs, mane and tail, with no pattern factor present other than white markings
Brown:
there is a mixture of black and brown pigment in the coat, with black limbs, mane and tail
Chestnut:
a chestnut may be any shade of red with no black points like the bay. Think of the different colours of a penny from brand new to very old and tarnished; chestnuts can come in all these colours
Also chestnuts may be described as follows if applicable:
- chestnut or sorrel with a flaxen mane and tail is a chestnut/sorrel colour body coat with a light coloured to almost white mane and tail
- dark chestnut is mahogany red
- light chestnut is light red to yellow
- liver chestnut is very dark red like a very old tarnished penny
- sorrel is yellowish to reddish to a brownish shade body coat. The mane and tail are usually the same or darker than the body
Cream:
the body coat is of a cream colour, with nonpigmented skin. The iris is deficient in pigment and is often devoid of it, giving the eye a pinkish or bluish appearance
Dun:
the body coat is cream colour with black mane and tail
Grey:
the body coat is a varying mosaic of black and white hair, with black skin. With advancing age, the coat grows lighter in colour. The flea-bitten grey may contain 3 colours or the 2 basic colours and should be so described. A pure white is exceptional
Palomino:
the body coat is a newly-minted gold coin colour (lighter or darker shades are permissible) with a white mane and tail
Piebald:
the body coat consists of large irregular patches of black and white. The line of demarcation between the 2 colours is generally well defined
Roan:
mixture of white hairs with 1 or 2 other hair colours in the coat. May be described as red roan (white and chestnut hair), blue roan (white and black hair) as applicable
Skewbald:
the body consists of large irregular patches of white and of any definite colour except black. The line of demarcation between the colours is generally well-defined
Strawberry:
the coat is chestnut with a mixture of white hairs
Unique coat marking additional identifying terms
Black marks or dark marks:
small areas of black or dark hairs occur together with the basic (usually lighter coloured) body colour hairs
Flecked:
small collections of white hairs occur distributed irregularly in any part of the body. May be further qualified as lightly flecked or heavily flecked depending on the amount of white hair
Grey-ticked:
white hairs are sparsely distributed through the coat or any specified part of the body
Leopard:
the term leopard may be added when the horse has many more or less circular collections of hairs differing from the general body colour
List:
a dorsal band of black hair which extends from the withers backwards to the base of the tail
Patch:
this term should be used to describe any larger well-defined irregular area (not covered by previous definitions) of hairs differing from the general body colour. The colour, shape, position and extent shall be described
Spots:
small, more or less circular, collections of hairs differing from the general body colour occur, distributed in various parts of the body. The position and colour of the spots must be stated
Withers stripe:
zebra band across the withers
Zebra marks:
dark or black striping on the limbs, neck or quarters. The affected part of the animal must be stated
White marks
The characteristics of all white marks must be described.
A white mark can be regular or irregular. It can be mixed with the hair of the coat, completely or in part, or at the edge. It can be bordered, a band of black skin shows under the white hair at the edge of the mark (the area appears bluish).
Head
The description should begin at the forehead, followed by the nasal bone, the muzzle, lips and chin.
Blaze:
a white marking covering almost the whole of the forehead between the eyes and extending beyond the width of the nasal bones and usually to the muzzle. Any variations in direction, termination and any markings on the white shall be stated
Flesh mark:
lack of pigmentation. A flesh mark is described as such and not as a white mark. Black spots within the flesh mark are to be indicated. All lip markings, whether flesh marks or white marks, shall be accurately described
Snip:
an isolated white marking, independent of those already named, and situated between or in the region of the nostrils. Its size, position and intensity shall be specified. When a snip is connected with a stripe it shall be recorded as such, for example star, stripe connected snip
Star:
any white mark on the forehead. Size, shape, intensity, position and coloured markings (if any) on the white to be specified. Should the markings in the region of the center of the forehead consist of a few white hairs only, it shall be so described and not referred to as a star
Stripe:
the narrow white marking down the face not wider than the flat anterior surface of the nasal bones. In many cases, the star and stripe are continuous and should be described as star and stripe connected. When the stripe is separate and distinct from the star it shall be described as interrupted stripe. When no star is present the point of origin of the stripe shall be indicated. The termination of the stripe and any variation in breadth, direction and any markings on the white shall be so stated, for example broad stripe, narrow stripe, inclined to left, etc. Any markings in the white area shall be stated
White face:
when the white covers the forehead and front of the face, extending laterally towards the mouth. The extension may be unilateral or bilateral, in which case it shall be described accordingly
White muzzle:
when the white embraces both lips and extends to the region of the nostrils
Limbs
All white markings on the limbs must be accurately defined and the upper limit precisely stated with reference to points of the anatomy, for example white to mid-pastern, white to upper third of cannon. The use of such terms as "sock" or "stocking" are not acceptable. The exact location must be specified, examples are listed below:
- white coronet, white pastern, white fetlock, white to knee, white to hock, white to hind quarter
- white patch on coronet (anterior, lateral, medial, posterior)
- white ring around limb: does not extend down to the coronet
The presence of coloured spots in white marks shall be recorded. Black spots in a white coronet are referred to as Ermine marks.
Hoofs:
any variation in the hoof pigment shall be noted
Whorls – Cowlicks
Whorls or cowlicks are changes in the hair pattern, and may take various forms simple, tufted, feathered or sinuous. Their position must be clearly specified with an "x" at their location on the horse.
Illustrations of white markings
Equine face markings
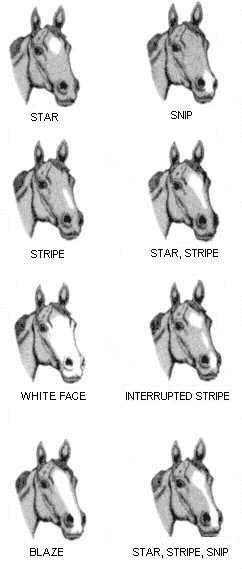
Description of the illustration of the Equine face markings:
8 pictures showing equine face marking: star, snip, stripe, star stripe, white face, interrupted stripe, blaze, star strip snip.
Equine legs markings
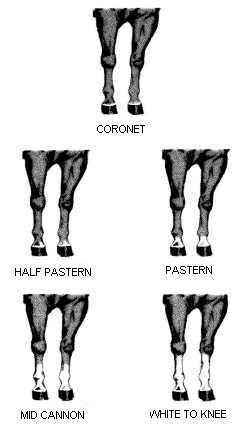
Description of the illustration of the equine legs markings:
5 pictures showing equine legs marking: coronet, half pastern, pastern, mid cannon, white to knee.
The silhouette
As described in section 2, a silhouette properly filled may be used in lieu of picture identification.
- General
- the silhouette must be filled in using both a red ballpoint pen and a black ballpoint pen
- blue ink must never be used because it is difficult to photocopy
- inks which run must be avoided (for example felt pens, ink pans)
- coloured pencils which can be erased must not be used
- the ballpoint pen used must have a broad point
- always use block letters
- Procedure
- the narrative should be completed first using a black ballpoint pen, followed by the silhouette indicating all the distinctive marks
- ensure that the silhouette and the narrative agree
A careful check must be made to ensure that all reference to left and right agree and no ambiguity exists.
- Red ballpoint pen
Everything which appears in white on the horse must be shown in red on the silhouette.
Bordered marks:
a white bordered mark has a definite outline, which is bluish and corresponds to the black skin under the white hairs. Bordered markings are indicated by a double lineFew white hairs:
few white hairs or grey-ticked areas are indicated by single short linesMixed marks:
mixed hairs are indicated by cross-hatchingUnpigmented areas:
unpigmented areas such as flesh marks, wall-eyes, or stripes on the hoofs are entirely coloured in redWhite marks:
white marks must be clearly outlined, with irregularities indicated, and without shading but lightly hatched-in if desiredWhite patches:
large white patches on piebald or skewbald horses should be cross hatched-in or line-shaded to differentiate them from other patchesVarious:
- the presence of white hairs in the mane and tail should be indicated with red lines
- permanent white marks in the coat acquired through trauma, freeze branding, surgery etc. should be indicated in the silhouette as for other white marks and by an arrow pointing at their location
- Black ballpoint pen
Identifying markings which are not white on the horse must be shown in black on the silhouette.
Black spots and marks:
black spots or marks on the coat or within a white mark or flesh mark must be outlined in black and left unshadedBrand marks:
brand marks should be drawn in black; if the shape is not visible the brand is to be considered as a scar and indicated by an arrowScars:
scars due to surgery, treatment or accidents are indicated by arrows pointing at their locationWhorls:
whorls are indicated by an "x", if the whorl is elongated, it is shown by a continuous line from the "x". The exact location of the whorls is very importantZebra marks, wither stripes and lists:
zebra marks, wither stripes and lists are indicated by thick black lines following the mark(s)
4. Examination/assessment of the EID
Information documents (individual EID/lot EID) represent the means of information transfer required under the SFCR, from the equine owner to the operator of the establishment that is identified in the licence for the activities and the CFIA.
Equine owner responsibilities
The equine owner is responsible for EID and must ensure that:
- individual EIDs are submitted to the operator with the equine animal or the lot EIDs are submitted as outlined in section 5 of this document
- all EIDs are complete, accurate and provide at least 180 days of history for the animal or animals presented for slaughter
- equine animals with a historical record of non-permitted drug usage (section 6) must not be presented for slaughter as food animals
- animals represented by EIDs sent to slaughter have met all medication related withdrawal periods as shown in sections 7 and 8, or on the product label, and/or have met withdrawal periods provided via a licensed veterinarian, through a veterinary/client/patient relationship, who has enlisted and cited the aid of a body recognized by the veterinary community as being capable of determining medication related withdrawal periods such as the Canadian Global Food Animal Residue Avoidance Databank (CgFARADTM) or a veterinary pharmaceutical department of an accredited Veterinary Medical College. In the case of a withdrawal determination, the withdrawal period/interval information provided from the recognized veterinary body must be attached to the EID
- equine animal with uncertain hazard status for physical, chemical or other contaminants are reported to the operator
- EIDs are signed to confirm they have been reviewed for completeness and accuracy.
The following 2 options are possible:- the owner identified in Part 2 of the EID has clear knowledge of the medical history pertaining to the equine represented by the EID
- In this case the owner must review the identification and medical history portions of the EID for completeness and accuracy and sign the owner's attestation in Part 3 of the EID or
- the owner identified in Part 2 of the EID has entrusted the care of the equine identified on the EID to another trusted person.
- In this case the entrusted person must have clear knowledge of the medical history pertaining to the equine represented by the EID and review the identification and medical history portions of the EID for completeness and accuracy
- This entrusted person must clearly include their contact information (that is, at least, name, title [for example: trainer], address and phone number) under the signature block in Part 3, and may sign the owner's attestation in lieu of the identified owner (Part 2)
- the owner identified in Part 2 of the EID has clear knowledge of the medical history pertaining to the equine represented by the EID
Operator responsibilities
EIDs provide the operator with the necessary level of confidence that identified potential biological, chemical and physical hazards associated with live equine animals have been recorded, and to the extent possible, prevented or controlled at the farm level and during transportation. The operator must notify the CFIA immediately in the event of any discrepancy(s).
- Every operator must develop, implement and maintain a control program within their PCP that ensures the validity and accuracy of information contained on EIDs they accept via procedures such as the provision of information to transient agents related to enhancing the accuracy of EIDs, transient agent training, and supervision of the transient agent business procedures including periodically contacting previous owners via the contact information provided on EIDs to ensure accurate information was captured
- The control program must contain contact information for every transient agent doing business with the operator
- The operator must develop and implement effective corrective action(s) and take appropriate measures on animals and meat products as required in cases of non-compliance
- The operator must develop and implement a control procedure that will link and maintain the identity and associated information between the EID/lot EID and the animal's carcass through the slaughter process including carcass cooling, to facilitate trace back if required
- The operator is responsible for evaluating all submitted individual animal EIDs and lot EIDs as part of the ante-mortem examination (screening) process, to determine if they are complete and accurate and to ensure the animals they represent are fit for slaughter
- If the documents are accepted by the operator, the documents (or copies thereof) and results of exam are submitted to the CFIA in order to perform a complete ante-mortem examination
- If the information document is incomplete the operator may contact the equine owner to obtain a complete information document
- In the case of sub lot equine information document (SLEID) submission, the operator must ensure that the CFIA has the original or a copy of the relevant initial equine information document (IEID) for a correlation comparison
- After CFIA assesses EIDs, the operator must file all information documents and keep them for a minimum of 1 year
- Information document records must be made available to the CFIA upon request
- In order to facilitate the CFIA verification activities at these different locations, slaughter plant operators will have a signed agreement from the owners and equine buyers presenting equine for slaughter at their facility accepting potential CFIA verification activities on premises holding equine
- The operator must provide the names and contact information of all owners and transient agents to the CFIA upon request
Missing or incomplete EIDs
Operators may choose 1 of the following 3 options, after notifying the CFIA, for equine that arrive at their establishment for slaughter without the appropriate or fully completed information document:
- first option
- If assured that a fully completed EID will arrive, the operator may elect to hold the animal(s) in compliance with section 137 of the SFCR
- Compliance with animal welfare requirements is the responsibility of the operator
- The veterinarian with supervisory authority will evaluate the complete faxed/electronic copy of revised information documents
- (Note that live animals imported into Canada as "slaughter only" are subject to time restrictions under the Health of Animals Act and Health of Animals Regulations limiting the amount of time they may remain alive in Canada) or
- second option
- Slaughter the equine animal and hold all meat products and by-products derived from it until the operator obtains documentation, accepts it and submits its exam results with the documentation to the CFIA or
- third option
- Humanly kill the equine as a segregated lot and treat all derived meat products and by-products as inedible meat products (The operator must ensure that potential chemical residues and veterinary drugs issues do not implicate and restrict the use of inedible material)
The CFIA's role
The CFIA's assessment of the EIDs reviewed by the operator allows CFIA staff to judge whether or not if the operator is implementing all necessary corrective actions and preventive measures when evaluating incoming animal information in accordance with the SFCR.
- a CFIA veterinarian will assess the EIDs to ensure that all the necessary information (identification, medical history/compliance of substances and withdrawal periods, declarations) is complete, that the previously submitted animal identification method is used for lot EIDs and that the SLEID identity information correlates to a previously submitted IEID
- once ante-mortem inspection is completed and it is determined that the animals may proceed to slaughter, the CFIA veterinarian will sign the CFIA/ACIA 1438 or equivalent in-house form to authorize slaughter.
- No equine shall proceed to slaughter unless the associated CFIA/ACIA 1438 or equivalent in-house form is signed by the CFIA
- a sufficient number of EID and/or lot EID is selected by the CFIA for identity verification purposes to establish comfort over the operator's ante-mortem identification check
- the CFIA may assess the effectiveness of the operator's control over the validity and accuracy of EIDs at the industry level
- the CFIA may contact equine owners and transient agents presenting equine for slaughter and conduct assessment of the means of record keeping, EIDs creation, maintenance and transfer including onsite verification
4.1 Verification of the equine slaughter operator's control over the validity of EIDs
Industry verification
Operators of equine slaughter facilities utilize transient agents (buyers) to obtain the vast majority of equine for slaughter. The transient agents (buyers) obtain equines at public auction and from private sale. Transient agents (buyers) and livestock auction market personnel are key players in the creation and transfer of EIDs. It is imperative that the information provided by equine owners and captured in the owner signed EID provides an accurate medical history and identification of the animal. To ensure an accurate EID is provided to the operator, operators must ensure equine owners and transient agents follow procedures outlined in this document, the operator's PCP.
To evaluate the operator's control over the validity of the information provided through EIDs, the CFIA will conduct verifications of the EIDs creation, update, record-keeping and transfer process. The CFIA verification evaluations apply to owners, buyers, records and equine under the care/control of equine buyers or private owners at assembly points and premises; at public auction; and at private sale premises, via an agreement having been previously signed by the operator with the various stakeholders. The CFIA has created specific tasks to guide and track CFIA verifications.
Reporting industry deficiencies and follow-up
Deficiencies found during the CFIA verification activities listed above may indicate a lack of effective operator control over the validity of EIDs. As such, the verifying CFIA inspector must contact the veterinarian with supervisory authority at the implicated operator(s) facility and provide the details of deficient findings. The veterinarian with supervisory authority or designate, provides the details of the finding to the plant operator. The operator must take appropriate compliance action related to implicated animals, or product if necessary, as well as implement/enhance their ante-mortem preventive control program and procedures as required to enhance/assure the validity of EID information. The CFIA will conduct a verification of the operators control procedures and corrective actions related to the validity of EIDs using the appropriate task.
Residue finding follow-up procedures related to EID validity
Chemical residue results that indicate residues detected in equine meat products must be investigated by the operator to determine if the implicated previously accepted EID contains declarations that may not be valid. The corresponding EID must be located by the operator. If discrepancy exists between declared drug usage and residues detected, the operator must effect corrective actions to ensure the validity of EIDs they accept is strengthened. The CFIA will conduct a verification of the operators control procedures and corrective actions related to the validity of EIDs accepted by the operator.
Measures to strengthen the EID traceability system for equine imported into Canada from the United States
A certificate, namely the Equine Certification Document (ECD), will be required for equine exported to Canada for slaughter or feeding purposes from the United States. The US accredited veterinarian will review the EID representing each equine to be exported to Canada for slaughter or feeding at the time of export certification. The ECD must be generated and signed by a veterinarian accredited within the United States Department of Agriculture (USDA) to perform equine export certification duties. The ECD must accompany export documentation to the slaughter establishment for slaughter horse importations or to the feedlot for feeder horse importations.
The Equine Certification Document must state:
I ![]() have verified that all equine included on the Export Health Certificate number:
have verified that all equine included on the Export Health Certificate number: ![]() , are accompanied with an Equine Information Document (EID) properly completed and signed by the owner.
, are accompanied with an Equine Information Document (EID) properly completed and signed by the owner.
Number of EIDs that accompany the Health Certificate: ![]()
![]()
Signature of accredited veterinarian
![]()
Date
Business contact information of the accredited veterinarian including business name, address and phone number.
![]()
![]()
It is strongly recommended that the US accredited veterinarian utilize the statement above on their veterinary company letterhead which provides their company name, address and phone number to generate the Equine Certification Document (ECD).
5. Equine Lot Program
An owner of a group(s)/lot(s) of equine animals intended as food animals may be eligible to present the animals to slaughter via a collective lot declaration.
In order to avoid confusion with any other method of identification, the method of identifying the Equine Lot Program must be unique to each lot owner and for each lot (for example: owner's ID and lot ID »» XYZ 2021-1). This method must be submitted to the operator for examination and approval and presented to the veterinarian with supervisory authority for assessment of program compliance (which includes the SFCR's requirements) prior to use. Failing to do so will lead to rejection of the lot at the time of slaughter.
There are a number of advantages to the lot program:
- each of equine held in a lot established under an Equine Lot Program will not be required to be identified by a full narrative description and pictures on an individual EID when presented for slaughter
- the review of records prior to slaughter and the amount of paperwork to be retained on file is greatly decreased
- the CFIA's risk-based inspection approach recognizes factors that may impact food safety risk. Equine enrolled in the Equine Lot Program are subjected to greater veterinary oversight and are deemed a lesser food safety risk
Standards for establishing a group (lot) of equine intended for slaughter
- Lot owner responsibilities
The owner of any proposed lot of equine intended for slaughter must ensure that:
- a lot identification method(s) has been submitted to the CFIA and meets the requirements
- a letter of commitment is provided to the operator of the slaughter establishment that is identified in the licence for the activities, as described in the lot identification examination/assessment section
- a lot control program is established that associates history information to identified lots
- a drug and vaccine use program including drug withdrawal period information is developed and health information is recorded
- arrangements are made with a licensed veterinarian to verify the Lot Program and perform onsite verification activities
- the IEID and SLEID (or equivalent documents that meet CFIA requirements) be submitted, as required by this policy, to the operator of the establishment that is identified in the licence for the activities
- any changes made to the method(s) of lot identification must be submitted to the operators for acceptance and presented to the CFIA for reassessment prior to implementation
- verification assessment findings are corrected in a timely manner and
- records are kept and maintained in a timely and auditable manner
- Operator responsibilities
Operators are to work with potential lot owners and the CFIA to ensure that a system of animal identification and in plant procedures enable the operator to maintain traceability throughout the slaughter process. Procedures and methods of identification from live animal receiving to processing must be established to ensure that meat products may be traced back to an implicated lot.
Operators are to perform ante-mortem examination as per the document: Standards for ante-mortem examination and inspection, which includes a review of EIDs for acceptability by the operator and presentation of their results to the CFIA. The operator must adjust his or her Preventive Control Plan (PCP) as needed.
- CFIA responsibilities
The CFIA is to assess a potential lot owner's lot identification requests to ensure the method is unique and effective to maintain traceability. When the initial lot identification request is found acceptable to the operator, it is assessed by the veterinarian with supervisory authority.
Equine Lot Program elements
The prospective lot owner will submit written details of the proposed method of lot identification they wish to use to ensure uniqueness, traceability and compliance to the SFCR. The method must receive approval from the operator and meet CFIA requirements prior to use.
- Unique lot identification method
The lot owner may apply for an identification method that identifies a selected lot of equine animals intended for slaughter. At the time that a lot of equine animals is established as a pre-slaughter lot, the unique lot identifier must be applied to each animal of the lot.
- Unique individual animal lot identification method
The lot owner may apply for a unique individual animal identification method for each equine intended to be presented as a lot at slaughter.
- Lot identification examination/assessment
The examination process involves an initial review and acceptance by the operator, and an exam by the CFIA veterinarian with supervisory authority of the establishment to which the animals will be shipped, prior to use of the identification method.
A letter of commitment from the potential lot owner to the operator of the slaughter facility and the veterinarian with supervisory authority must also be submitted. In the letter of commitment, the lot owner must give assurances that he or she understands the requirements outlined in this document. The letter of commitment must also include a statement that the lot owner is aware of, and accepts that the lot program, animals and premise are subject to audit activities coordinated by the CFIA.
The lot identifier must be capable of being maintained for the time period the lot is expected to be held prior to slaughter. Any animals added to a lot must have the lot identifier applied upon entry into the lot.
Owners may request to identify lots of equine animals under either of the 2 options above or both options when more than 1 lot is owned. In the case of equine animal identified by both a unique lot identifier and a unique animal identifier, the unique animal identification method procedures and requirements apply.
- Lot control program and record
A documented lot control program must be established by the owner of a lot of equine animals. The lot control program will ensure that medical records correlate to the animals specified within a declared lot. The lot owner will ensure that animals are not inadvertently added to any specific lot without ensuring the added animals have a medical history compatible with the lot they enter and are identified according to other animals of the lot they enter.
Records of animals contained within each lot intended for slaughter must be established upon creation of the lot and brought up to date/verified as animals are removed from the lot, added to the lot, determined to be missing from the lot, and shipped for slaughter. Lot control records must also include the date of creation of the lot, the date the lot would be eligible to be slaughtered considering the requirement for at least a 180 day recorded history prior to slaughter for all animals within the lot, the unique lot identifier or animal identification information, and entries that indicate the date or dates animals from the lot were shipped for slaughter and the number of animals in each shipment.
Animals may be added to an established lot only if they are accompanied by completed and compliant EID(s) (that is, compliant non permitted drug use history, withdrawal periods have been met or will be met prior to slaughter, identity is confirmed) that are compatible with the start date established for the lot they enter. These EID(s) must be filed by the lot owner and made available for inspection/verification with other information applicable for the established lot they enter.
For any animal removed from an established lot but not sent for immediate slaughter, the lot owner has the following 3 options:
- an individual EID is created by the lot owner or
- the animal is moved to a new lot created with a new projected slaughter date that adheres to at least 180 days of recorded compliant drug use history prior to slaughter and the lot identifier for the new lot is applied to the animal upon entry into the new lot or
- the owner has already an unique individual animal identification method that meets CFIA requirements, identifies the animal individually and enrols the animal into an individual animal lot program
Each record event entry in lot control records must be accompanied by the initials or identification of the person making the entry and date/time of the entry.
Lot control records and supplemental information associated with the animals in the lot such as previous EIDs must be accurate and kept up to date in a timely manner as well as maintained on file by the lot owner for verification and oversight purposes from the time the lot is established until 2 years after all animals of the lot have been fully shipped for slaughter.
- Drug and vaccine program and lot record
The lot owner must prepare a drug and vaccine program which lists the drugs and vaccines permitted to be given or fed to equine enrolled in a lot program. The drug and vaccine program must provide the brand name of the drug and/or vaccine permitted for use, the predetermined prior to slaughter withdrawal period associated with the use of the specified drug or vaccine, and the source of the withdrawal period information for each drug or vaccine listed.
Records of drug and/or vaccine use are established upon creation of a lot and maintained in a timely manner. Individual animal drug and vaccine use records are required for each animal identified via the unique individual animal lot identification method. In the case of unique lot identification method, a record of drug and vaccine use for the lot is to be maintained. All medication used for individual animals remaining within an established lot under the unique lot identification method will need to be declared for the entire lot.
The record of drug and vaccine use must contains:
- the date of lot creation (for unique lot method only)
- the first date the lot animal(s) may be shipped for slaughter considering the requirement for at least a 180 day recorded history prior to slaughter
- the drug identification number (DIN) or equivalent unique identifier, if any
- record entries that indicate the name of drug or vaccine used on any animal remaining in the lot
- the last date of use of any drug or vaccine on any animal remaining in the lot
- the number of animals treated
- the dose (amount of drug/vaccine) used
- the route of administration and
- the withdrawal period for the medication used, and the source of the withdrawal period information
Each record event entry is accompanied by the initials of the person making the entry and date/time of the entry.
Drug and vaccine lot records must be kept current and maintained on file by the lot owner for verification and oversight purposes from the time the lot is established until 2 years after the lot is fully shipped for slaughter.
- Lot health record
The owner of a lot of equine intended for slaughter must create and maintain a record of any disease or syndrome that was diagnosed or a description of any deviation from normal behaviour, physiology or appearance for any animal contained within each established lot. Individual animal health records are required for each animal identified via the unique individual animal method. In the case of unique lot identification, a single health record for the lot is to be maintained. Any disease/syndrome/deviation detected in a lot animal remaining within an established lot under the unique lot method will need to be declared for the entire lot.
Health records must contain:
- the date the lot was established (if using the unique lot method)
- the first date the animal(s) may be shipped for slaughter considering the requirement for at least a 180 day recorded history prior to slaughter
- the unique identifier
- record entries that indicate the date the disease/syndrome/deviation were noticed
- the details of the disease/syndrome/deviation
- the number of animals affected and
- date the disease/syndrome/deviation were resolved
Each record event entry is accompanied by the initials of the person making the entry and date/time of the entry.
Health records must be kept up-to-date in a timely manner and maintained on file by the lot owner for verification and oversight purposes from the time the lot is established until 2 years after the lot is fully shipped for slaughter.
- Verification review procedure
The lot owner must make arrangements for a licensed veterinarian to evaluate the general health status, medication use, identification and supporting documents/records pertaining to equine involved in a lot program. This evaluation is referred to as a veterinary verification. The lot owner must have sufficient proof of a valid veterinary/client/patient relationship. The veterinary verification must occur at least once every 6 months. Any costs associated with the lot program are the responsibility of the lot owner.
The licensed veterinarian will assess if the lot control and the drug and vaccine program are effective and/or being implemented as written. The licensed veterinarian will assesses if the lot control records, drug and vaccine records, and health records are being established as required and are complete, up-to-date and accurate. A review of supporting information such as previous owner EIDs is also conducted.
The licensed veterinarian will compare a sufficient number of IEIDs and SLEIDs to drug and vaccine use records as well as health records on file to ensure that the lot owner or designate is accurately transferring the on file information to the IEIDs and SLEIDs.
The licensed veterinarian will assess if the unique identifier is being applied as specified, is functional/legible, and is being retained on lot animals. If animals have been added to a lot, identity and lot standards for these animals are confirmed through identity verification (comparing the previous owner EID to the animal and then ensuring the unique identification method has been applied) and file maintenance verification (the correct records are being kept) of a sufficient number of these animals to provide confidence that there are no non-compliances. The licensed veterinarian will also assess the lot premise to identify potential food safety issues.
The licensed veterinarian performing the verification must officially document each assessment and findings including his or her name, signature and assessment date. Any deficiencies found must be noted by the verifier. The lot owner or designate must then ensure deficiencies are corrected in a timely manner, noted on the appropriate record as completed and signed off after completion including applicable initials/time/date of the record entry. The licensed veterinarian performing the verification will assess the effectiveness of any required corrections on the next verification or visit. The lot owner must retain a copy of the veterinary assessment on file for oversight purposes. A template checklist for this verification may be obtained from the CFIA.
The licensed veterinarian performing the verification must notify the operator of the establishment that is identified in the licence for the activities and the CFIA veterinarian with supervisory authority in the case of deficiencies that may impact the eligibility of a lot that has been or will be slaughtered.
- CFIA oversight
It is expected that the CFIA will perform an audit of the Equine Lot Program at least once per calendar year. The CFIA will perform the duties outlined under the verification review procedure section as well as audit the frequency and effectiveness of the assessments performed by the licensed veterinarian. A record of the CFIA oversight is created and a copy kept on file by the lot owner. A template checklist for this verification may be obtained from the CFIA.
Any deficiencies will be identified in writing to the lot owner and veterinary verifier for correction by the lot owner as indicated in the verification section above. The veterinary verifier will assess the correction on the next veterinary verification.
- Document submission prior to slaughter for equine identified by the lot methods
The following procedures are meant to minimize the likelihood and potential complications involved with receiving multiple animals at a slaughter plant with unsatisfactory documentation or pre-slaughter history.
The lot owner or designate must ensure that drug and vaccine lot records are current and complete, that all withdrawal periods have been met, that no not permitted drugs have been used and that the minimum slaughter date has been reached for any animals of a lot of equine shipped for slaughter on the day of shipment.
At least 3 working days prior to the expected date of slaughter of the first animal of a lot, the owner or designate must review and transcribe all relevant drug and vaccine use details as well as the health history applicable to the lot on file at the premise to the IEID as specified. The lot owner or designate will then (at least 3 days prior to the expected slaughter date) fax or electronically submit signed copies of the IEID to the operator of the establishment that is identified in the licence for the activities.
A SLEID carrying contact information, an original signature of the owner or designate and signature date is provided to the operator upon arrival of each truck/trailer carrying equine corresponding to the IEID previously sent to the slaughter plant.
Each individual animal identifier must be listed on the IEID and corresponding SLEIDs when animals are identified with unique individual animal identifiers.
The CFIA and the operator will exam the IEID and the SLEID to evaluate the eligibility of the animals for slaughter. Useful links: Standards for ante-mortem examination and inspection and ante-mortem Examination and Presentation Procedures for Food Animals
The owner or designate of the lot must make copies of, and keep on file at the premise, all IEIDs and SLEIDs sent to operators. These IEIDs and SLEIDs are subject to review during veterinary verification and CFIA oversight procedures.
- Alternate or equivalent document to IEIDs and SLEIDs
The operator of the establishment that is identified in the licence for the activities may apply to the CFIA for an exemption to the submission of IEIDs and SLEIDs from a specified lot owner as above, instead submitting 1 customized form that meets the CFIA's requirements, covering animals sent to slaughter on a specific day.
The operator must submit the customized lot EID template to the CFIA to ensure that it meets the requirements prior to use. The operator will provide the CFIA with a signed and dated letter stating that they are willing to accept the proposal from the designated premises to forego the submission of IEIDs and SLEIDs and that the operator understands that the exam and assessment procedures pertaining to a single lot EID may increase the possibility of holding or rejection of lots of equine animals prior to slaughter.
The proposed single lot EID must include all the information contained in both the IEID and SLEID. Single lot EIDs must be sent to a specified slaughter establishment from a specified Equine Lot Program premises.
In the case of multiple truck loads arriving at the slaughter establishment during a 24 hour period from a specified premise, the first truck load carries and presents the customized lot EID to the operator for review and acceptance and the CFIA for assessment prior to slaughter as per ante-mortem procedures.
6. List of veterinary drugs not permitted for use in equine slaughtered for food with Canadian brand name examples (March 10, 2010)
| Non permitted drug name | Examples of brand or common names | Species indicated on the label |
|---|---|---|
| 5-Nitroimidazoles including dimetridazole, metronidazole, and ronidazole | Banned by regulations Table Note 4 for sale in food producing animals in Canada. Not approved for veterinary use in Canada. |
N/A |
| Antibiotics used for growth promotion purposes such as olaquindox, carbadox, and tylosin | Carbadox not currently marketed in Canada (stop sale order in effect) Olaquindox not approved for veterinary use in Canada |
N/A |
| Antibiotics used for growth promotion purposes such as olaquindox, carbadox, and tylosin | There are no antimicrobials approved for use as growth promotants for equine in Canada. Equine animals treated with antibiotics for growth promotion reasons are not eligible for slaughter in Canada. | Several antimicrobials (for example, bacitracin, bambermycin, chlortetracycline, lincomycin, procaine penicillin, tylosin, virginiamycin etc.) have label claims for growth promotion/feed efficiency in other food producing animals (for example, cattle, swine, poultry). |
| Aristolochia species and preparations thereof | Not approved for veterinary use in Canada. | N/A |
| Arsanilic acid |
|
Chicken, turkey and swine |
| Arsanilic acid |
|
Turkey |
| Arsanilic acid |
|
Chicken and turkey |
| Beta-agonists used for growth promotion purposes, including clenbuterol and ractopamine | Clenbuterol Banned by regulations Table Note 4 for sale in food producing animals in Canada. |
N/A |
| Beta-agonists used for growth promotion purposes, including clenbuterol and ractopamine | Ractopamine
Zilpaterol hydrochloride
|
Cattle |
| Beta-agonists used for growth promotion purposes, including clenbuterol and ractopamine |
|
Swine |
| Beta-agonists used for growth promotion purposes, including clenbuterol and ractopamine |
|
Horses not intended for food |
| Boldenone |
|
Horses not intended for food |
| Chloramphenicol | Banned by regulations Table Note 4 for sale in food producing animals in Canada.
|
Dog and cat |
| Chloroform | Approved as a veterinary drug in Canada, however currently not manufactured | N/A |
| Chlorpromazine | Not approved for veterinary use in Canada. | N/A |
| Colchicine | Not approved for veterinary use in Canada. | N/A |
| Dapsone | Not approved for veterinary use in Canada. | N/A |
| Estradiol (for estradiol containing implants, see steroidal hormones below) |
|
Horses not intended for food, cattle, dog and cat |
| Estradiol (for estradiol containing implants, see steroidal hormones below) |
|
Cattle |
| Estradiol (for estradiol containing implants, see steroidal hormones below) |
|
Horses not intended for food |
| Methandriol | Not approved for veterinary use in Canada. | N/A |
| Nitrofurans including furaltadone, furazolidone, nitrofurantoin, nitrofurazone | Banned by regulations Table Note 4 for sale in food producing animals in Canada. Furazolidone not approved for veterinary use in Canada. Furox Aerosol Powder, Topazone Aerosol Powder, Furall registered for veterinary use in the USA. Furaltadone not approved for veterinary use in Canada |
N/A |
| Nitrofurans including furaltadone, furazolidone, nitrofurantoin, nitrofurazone |
|
Horses not intended for food |
| Nitrofurans including furaltadone, furazolidone, nitrofurantoin, nitrofurazone | Nitrofurantoin
|
Horses not intended for food, dog and cat |
| Nitrofurans including furaltadone, furazolidone, nitrofurantoin, nitrofurazone | Nitrofurazone
|
Horses not intended for food |
| Nitrofurans including furaltadone, furazolidone, nitrofurantoin, nitrofurazone |
|
General use |
| Nitrofurans including furaltadone, furazolidone, nitrofurantoin, nitrofurazone |
|
Dog and cat |
| Phenylbutazone |
|
Note: all of the products listed carry an indication for use in equine (but not equine intended to be slaughtered for food) |
| Resorcylic acid lactones including zeranol | Zeranol
|
Beef Note that this product carries only a cattle indication |
| Stanozolol | No active products for veterinary use in Canada. | N/A |
| Steroidal hormonal implants used for growth promotion purposes | Equine animals treated with steroid containing hormone implants used to promote growth are not eligible for slaughter in Canada. | Note that these products carry only a cattle indication. Hormonal implants containing estradiol or melengestrol acetate singly, or the combinations of estradiol and progesterone; estradiol and testosterone; estradiol and trenbolone acetate etc. sold under different brand names for use in cattle. |
| Stilbenes, stilbene derivatives, and their salts and esters including diethylstilbestrol | Banned by regulations Table Note 4 for sale in food producing animals in Canada. Diethylstilbestrol
|
Dog and cat |
| Testosterone |
|
Horses not intended for food |
| Thyrostats, antithyroid agents administered under any circumstances for the purpose of growth promotion | Approved for use in humans. Use in animals would be under veterinary control, but animals treated with these substances would not be eligible for slaughter. | N/A |
N/A: Not applicable as these active ingredients are not approved for veterinary use in Canada.
7. List of "essential" veterinary drugs permitted in equine with a 180 day withdrawal period with Canadian brand name examples
| Drug use | Drug | Canadian brand name examples |
|---|---|---|
| Analgesia |
|
No known manufacture for veterinary use in Canada |
| Antimicrobials |
|
Amiglyde-V |
| Antimicrobials |
|
No known manufacture for veterinary use in Canada |
| Antiprotozoal |
|
No known manufacture for veterinary use in Canada |
| Antiprotozoal |
|
Quinnoxine-S Sulfaquinoxaline-S |
| Cardiovascular |
|
No known manufacture for veterinary use in Canada |
| Convulsions |
|
No known manufacture for veterinary use in Canada |
| Fungal infection |
|
No known manufacture for veterinary use in Canada |
| Fungal infection |
|
Conofite Cream 2% Dermazole Shampoo Surolan Drops |
| Fungal infection |
|
Canaural Ear Drops Panalog Cream Panalog Ointment |
| Gastrointestinal |
|
No known manufacture for veterinary use in Canada |
| Hyperlipaemia |
|
Caninsulin |
| Hypotension or respiratory stimulation during anaesthesia |
|
Antihistamine Antihistamine Powder Antihist Solution Pyrahist-10 |
| Hypotension or respiratory stimulation during anaesthesia |
|
No known manufacture for veterinary use in Canada |
| Inhalation anaesthetics |
|
No known manufacture for veterinary use in Canada |
| Local anaesthetics |
|
No known manufacture for veterinary use in Canada |
| Muscle relaxants and associated substances |
|
No known manufacture for veterinary use in Canada |
| Muscle relaxants and associated substances |
|
No known manufacture for veterinary use in Canada for the use indicated |
| Ophthalmic |
|
Optimmune |
| Ophthalmic |
|
No known manufacture for veterinary use in Canada |
| Respiratory |
|
No known manufacture for veterinary use in Canada |
| Rhabdomyolysis |
|
No known manufacture for veterinary use in Canada |
| Sedation and premedication (and antagonism) |
|
Ace Acevet 10 Tablets Acevet 25 Tablets Acevet Injection Atravet 10 mg Injectable Atravet Soluble Granules |
| Sedation and premedication (and antagonism) |
|
Antisedan |
| Sedation and premedication (and antagonism) |
|
No known manufacture for veterinary use in Canada |
| Sedation and premedication (and antagonism) |
|
PropoFlo Rapinovet |
| Sedation and premedication (and antagonism) |
|
No known manufacture for veterinary use in Canada |
| Miscellaneous |
|
Chotin |
| Miscellaneous |
|
No known manufacture for veterinary use in Canada |
8. List of veterinary drugs safe for use in equine intended for food production for which withdrawal periods have been determined with Canadian brand name examples
Health Canada recommends the following provisional withdrawal periods (WP) for veterinary drugs in equine intended for food production. The following table will be updated periodically with the inclusion of new drugs or revised withdrawal periods, when additional information (for example, new data from the drug sponsor) becomes available. When the label recommended WPs are not specific to equine, Health Canada recommends using the provisional WPs listed in the following table.
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Amikacin |
|
Intrauterine | 6 months |
| Benzathine penicillin (in combination with procaine penicillin) |
|
Intramuscular (IM) | 60 days |
| Ceftiofur |
|
IM | 5 days |
| Gentamicin |
|
Intrauterine | 45 days |
| Neomycin | Neomycin (± astringents ± electrolytes ± anticholinergic):
|
Oral | 30 days |
| Neomycin and sulphonamide combinations | Neomycin and sulfonamides (± astringents ± electrolytes ± anticholinergic):
|
Oral | 30 days |
| Procaine penicillin |
|
IM | 28 days |
| Potentiated sulfonamides | Sulfonamide-trimethoprim (Oral):
|
Oral | 7 days |
| Potentiated sulfonamides | Sulfonamidetrimethoprim (Injectable):
|
Intravenous (IV), IM | 12 days |
| Sulfonamides Table Note 5 | Sulfonamides (± astringents ± electrolytes ± anticholinergic):
|
Oral | 12 days |
| Tetracycline |
|
Orale |
O: 5 days |
| Tetracycline |
|
Intrauterine | 18 days |
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Fenbendazole |
|
Oral | 13 days |
| Ivermectin |
|
Oral | 28 days |
| Ivermectin and praziquantel |
|
Oral | 28 days |
| Moxidectin | Quest Gel (Wyeth) | Oral | 36 days |
| Moxidectin and praziquantel | Quest Plus Gel (Wyeth) | Oral | 36 days |
| Piperazine | Powder/pellet formulations:
Liquid formulations:
|
Oral | 21 days |
| Pyrantel |
|
Oral | 7 days |
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Acepromazine | Oral formulations:
|
Oral | 6 months |
| Acepromazine | Injectable formulations:
|
IM, IV | 6 months |
| Butorphanol | Torbugesic (Wyeth) | IV | 7 days |
| Detomidine | Dormosedan (Orion/Pfizer) | IM, IV | 7 days |
| Lidocaine | Lidocaine Neat (Wyeth) Lurocaine (Vetoquinol) |
Subcutaneous (SC), IM | 7 days |
| Lidocaine and epinephrine |
|
SC, IM | 7 days |
| Romifidine | Sedivet (Boehringer) | IV | 14 days |
| Thiopental | Thiotal 1 G (Vetoquinol) Thiotal 5 G (Vetoquinol) |
IV | 7 days |
| Xylazine |
|
IM, IV | 35 days |
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Dexamethasone |
|
Oral, IV, IM | 21 days |
| Dexamethasone and trichlormethiazide | Naquasone (Schering) | IM | 21 days |
| Prednisolone |
|
IM, IV, Intra-articular | 28 days |
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Flunixin |
|
IM, IV | IV:10 days IM: 30 days |
| Ketoprofen | Anafen inj.100 mg/mL (Merial) | IM, IV | 7 days |
| Vedaprofen | Quadrisol 100 (Intervet) | Oral | 21 days |
| Vedaprofen | Quadrisol 50 Inj (Intervet) | IV | 21 days |
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Altrenogest | Regu-mate solution 0.22% (Intervet) | Oral | 42 days |
| Progesterone Table Note 6 | Progesterone 5% (Vetoquinol) | IM | 14 days |
| Drug | Approved Canadian products | Route | WP |
|---|---|---|---|
| Furosemide | Furosemide inj. (Sandoz) Salix inj. (Intervet) |
IM, IV | 7 days |
| Omeprazole | Gastrogard (Merial) | Oral | 3 days |
| Sodium iodide |
|
IV | 0 days |
| Trichlormethiazide and dexamethasone | Naquasone (Schering) | IM | 21 days |