On this page
- 1. Purpose
- 2. Sampling methods
- 3. Preparing for shipping
- 4. Laboratory standards to provide testing and results
- 5. Analytical methods
1. Purpose
This annex provides information to help food businesses collect and prepare samples for the Process Verification Monitoring Program (PVMP) for generic E. coli in raw poultry.
It's your choice!
You may use other sampling and testing methods developed by provincial governments, industry associations, international partners or academic bodies. Ensure to have the proposal verified by the Canadian Food Inspection Agency (CFIA) for foreign country equivalency assessment.
What is not included!
While this document provides specific details regarding sampling for E. coli in poultry, it does not describe general best practices for sampling. Be sure to also consult the document Sampling procedures.
2. Sampling methods
2.1 Key considerations when sampling
- Consider time sensitivity of the samples
To obtain the most accurate results, the laboratory should analyse samples within 24 hours of collection.
When this cannot be achieved, such as for statutory holidays or weekends, collect the carcass, control it under refrigeration and ship sample to the laboratory only when analyses can be guaranteed within 24 hours of collection.
- Remember the suitability of the samples: temperature
The laboratory will not analyze samples which are frozen (below 0°C) or too warm (with temperature greater than 10°C for rinsate).
If samples need to be sent off-site, ensure that samples are maintained at refrigeration temperatures until transport and shipped refrigerated to the laboratory performing the analysis. Avoid storing shipping containers near heaters or in areas exposed to excessive heat. Avoid freezing your samples.
- Be prepared for sampling: check that all supplies are suitable
- Prepare and label, as necessary, your sampling supplies ahead of sampling
- Consider assembling in a tote which will also carry supplies to sanitize the sampling station
- Verify your sampling medium: discard any Butterfield's phosphate diluent (BPD) solutions that are cloudy, turbid or contain particulate matter.
Refrigerate solutions 24 hours prior to use.
- Ensure templates are sterile and dry
Templates can be reusable or not. They can be made of metal, aluminum foil, brown paper, flexible plastic, etc.
- Have means to control your sample, such as a tamper proof seal, as soon as collection is completed
Note: sterile Buffered Peptone Water (BPW) can be substituted for Butterfield's phosphate diluent (BPD) however there may be a slight rise in bacteria counts following this change (¼ log increase). If a Process Verification Criteria (PVC) has been published for the sampling method, marginal ("m") and unacceptable ("M") limits are not allowed to be changed.
2.2 Carcass rinse method for all poultry species other than turkey and geese
Sampling supplies
- Sterile gloves
- 2 sterile 3500 milliliter (ml) sterile stomacher-type or self-sealing type bags or equivalent large enough to hold the carcass while rinsing
- 400 ml sterile, pre-chilled Butterfield's phosphate diluent (BPD)
- Plastic tie wraps or equivalent to secure the bag
- Optional - Sterile leak-proof container
Sample collection
- Use aseptic techniques described in the document Sampling procedures
- Select the sample, at the randomly selected time
- Identify the point in the process where the carcasses will be selected for sampling: carcass are to be sampled from the post-chill area after all interventions have taken place and after sufficient drip time
- select a carcass and then count back or ahead 5 carcasses and select the next carcass for sampling (random, unbiased selection)
- if the sixth carcass is not a whole carcass with intact skin, count back or ahead an additional 5 carcasses for sample selection
- repeat until a whole carcass is available
- in establishments where the end location of the drip line makes removing a carcass from a moving line unsafe, pull the sample at the chiller exit, directly from the conveyor belt ensuring sufficient drip time
- if the final antimicrobial intervention is temporarily displaced because of an unforeseen event such as equipment malfunction, select a carcass after the alternate final intervention step
- take the randomly selected carcass:
- open the sampling bag without touching the sterile interior.
Do not let anything but the sampled carcass touch the interior of the sampling bag
- put on sterile gloves
- grab the bottom exterior of the bag and push up through the bottom to form a "glove"
- grab the carcass by the leg; allow drainage of excess fluid to prevent dilution of the rinsate (such as allowing a drip time of at least 1 minute) taking care to avoid cross-contamination
- use other hand to pull the bag, from the exterior surface, back over the carcass
- open the sampling bag without touching the sterile interior.
- Prepare the sample
- place the bag with the carcass on the flat sanitized surface
- open the BPD container and pour the BPD over the carcass in the bag
- manipulate the loose neck skin over the neck bones through the bag
- expel the excess air from the bag, twist it closed, and fold the twist over
- while securely holding the closed bag, rinse the carcass inside and out using a rocking motion for approximately 1 minute.
This assures both interior and exterior of the carcass get rinsed.
- place the bag with the chicken on the sanitized flat surface with the top of the bag facing up
- remove the screw-cap from the sterile sample container, and put the cap in the small re-sealable sterile bag
- open the large bag with the chicken, and pour 30ml of the BPD liquid into the sterile sample container
- take the screw-cap out of small re-sealable bag, and close the sample container.
- ensure that the lid is correctly threaded, and do not over-tighten
- place sample container in the secondary zipper-lock bag, removing excess air
- discard the remaining liquid
- return the chicken to the chill tank or to location where the carcass was collected
Quick tip
Sampling can also be performed by 2 people wearing sterile gloves:
- 1 to hold the bag open
- 1 to grab the carcass, to pour the BPD
2.3 Carcass rinse method for turkey and geese
Note: an assistant is recommended for the sampling
Sampling supplies
- Sterile gloves
- 2 sterile 3500 ml stomacher-type or self-sealing type-bags or equivalent large enough to hold the carcass while rinsing.
- The bags size is approximately 18 inches × 24 inches.
- Large turkeys should be placed in a plain, clear polypropylene autoclave bag, about 24 inches × 30 inches to 36 inches.
- 600 ml sterile, pre-chilled Butterfield's phosphate diluent (BPD)
- Plastic tie wraps or thick rubber bands or equivalent, if needed to secure sample bag
- Optional - Sterile, leak-proof container
Sample collection
- Use aseptic techniques described in the document Sampling procedures
- Select the sample, at the randomly selected time
- Identify the point in the process where the carcasses will be selected for sampling: carcass are to be sampled from the post-chill area after all interventions have taken place and after sufficient drip time
- select a carcass and then count back or ahead 5 carcasses and select the next carcass for sampling (random, unbiased selection)
- if the sixth carcass is not a whole carcass with intact skin, count back or ahead an additional 5 carcasses for sample selection
- repeat until a whole carcass is available
- in establishments where the end location of the drip line makes removing a carcass from a moving line unsafe, pull the sample at the chiller exit, directly from the conveyor belt
- if the final antimicrobial intervention is temporarily displaced because of an unforeseen event such as equipment malfunction, select a carcass after the alternate final intervention step
- take the randomly selected carcass:
- the assistant opens the sampling bag without touching the sterile interior.
Do not let anything but the sampled carcass touch the interior of the sampling bag
- put on sterile gloves
- grab the carcass by the legs; allow drainage of excess fluid to prevent dilution of the rinsate (such as allowing a drip time of at least 1 minute) taking care to avoid cross-contamination
- place the turkey carcass, vent side up, into a sterile sampling bag
- while the assistant supports the carcass, manipulate the loose neck skin on the carcass through the bag and position it over the neck bone area to prevent puncturing of the bag
- the assistant opens the sampling bag without touching the sterile interior.
- Prepare the sample
- The assistant maintains bag open from the exterior while supporting the carcass
- open the BPD container and pour the BPD over the carcass in the bag
- take bag from assistant, expel the excess air from the bag, twist it closed, and fold the twist over
- while securely holding the closed bag, rinse the carcass inside and out using a rocking motion for approximately 1 minute.
This assures both interior and exterior of the carcass get rinsed.
- return bag to assistant
- with a gloved hand remove the carcass, taking care not to touch interior of bag and allowing excess liquid to drip back in the bag; return the carcass to the chill tank or to location where the carcass was collected
- expel excess air from bag, taking care not to expel any rinse fluid.
Secure the top of the bag so that the rinsate will not spill out or become contaminated. Alternately transfer at least 30ml of rinsate into a sterile sample jar.
- place sample container in the secondary zipper-lock bag
2.4 Carcass sponge method for turkey and geese
Sampling supplies
- Sterile gloves
- Sterile specimen sponge in sterile wire twist top type-bag or equivalent
- 25 ml sterile pre-chilled Butterfield's phosphate diluent (BPD)
- Sterile plastic self-sealing-type bag or stomacher-type bag
- 5 × 10 cm sterile template
Sample collection
- Use aseptic techniques described in the document Sampling procedures
- Select sample, at the randomly selected time
- identify the point in the process where the carcasses will be selected for sampling: carcasses are to be sampled from the post-chill area after all interventions have taken place and after sufficient drip time
- select a carcass and then count back or ahead 5 carcasses and select the next carcass for sampling to avoid any possible bias during selection
- if the sixth carcass is not a whole carcass with intact skin, count back or ahead an additional 5 carcasses for sample selection
- repeat until a whole carcass is available
- in establishments where the end location of the drip line makes removing a carcass from a moving lie unsafe, pull the sample at the chiller exit, directly from the conveyor belt
- if the final antimicrobial intervention is temporarily displaced because of an unforeseen event such as equipment malfunction, select a carcass after the alternate final intervention step
- take the randomly selected carcass; allow drainage of excess fluid to prevent dilution of the rinsate (such as allowing a drip time of at least 1 minute) taking care to avoid cross-contamination.
Do not touch the back or thigh areas.
- Prepare the samples using aseptic techniques:
- place the carcass on a rack or breast-down on paper towels
- do not let sample sites touch any surfaces
- open sponge bag and BPD bottle, taking care not to contaminate inside surfaces
- carefully pour about half of the contents of the sterile BPD bottle (10 ml) into the sponge bag and recap bottle
- close bag and massage sponge; squeeze sponge and push to top of bag
- open sponge and template bags and set bags on sanitized surface
- put on sterile gloves
- retrieve sponge and template aseptically
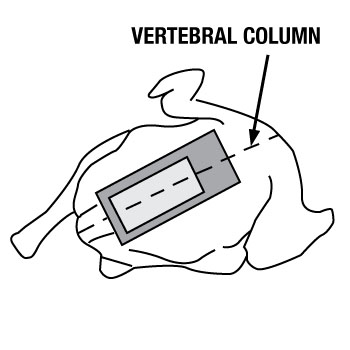
- position and hold the template just above the tail over the vertebral column (picture A), avoiding all contact to the sampling area
- wipe the sponge over entire sample area (10 vertical swipes and 10 horizontal swipes)
"Rolling" the template may be necessary during swabbing since the surface of the carcass is not flat
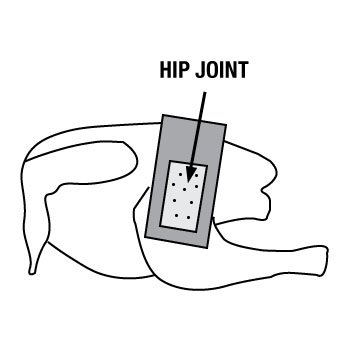
- reposition and hold the template over the thigh (lateral to hip - Picture B), avoiding all contact to the sampling area.
- turn sponge over and wipe unused side over entire sampling area (10 vertical swipes and 10 horizontal swipes)
- place the sponge in the sampling bag and seal the bag. do not touch the surface of the sponge to the outside of the sponge bag
- add the additional BPD about 15 ml to the bag to bring the total volume to approximately 25 ml
- press wire closures of the sponge bag together, expel excess air, then fold down the top edge of the bag 3 or 4 times; secure the bag by folding the attached wire tie back against the bag
- place sample bag in the secondary zipper-lock bag, removing excess air
- place bagged carcass sponges under refrigeration within 5 minutes of collection.
3. Preparing for shipping
Shipping supplies
- Sample seal for tamper proofing
- Absorbent pad
- Cardboard separator(s)
- Frozen gel coolant pack(s) (place in freezer 24 hours prior to shipping)
- Shipping container, with foam plug as applicable
Packaging procedure
- maintain sample security and identification
- place in the shipping container in the following order:
- the absorbent pad
- the frozen gel coolant pack
- the cardboard separator
- the sample collection jar or sample bag, within the secondary zipper lock bag, placed upright
Do not tape or wrap the sampling container, as laboratories may refuse the sample
- a second cardboard separator followed by a second frozen gel pack if there is added risk that the temperature of the sample could get compromised (for example summer heat, long transport time)
- the foam plug, if available, directly on top of the sample jar, pressing down slightly to secure contents.
Do not overfill the shipping container.
- place the insulated lid on the container
- seal the shipping container
- maintain shipping container under appropriate temperature control until pick-up
- ship the sample in order for laboratory analysis to be completed within 24 hours of collection
4. Laboratory standards to provide testing and results
To be eligible to provide testing and results, laboratories should be accredited by the Standards Council of Canada (SCC) or Canadian Association for Laboratory Accreditation Inc. (CALA) and have on their scope of approved methods, the method(s) of analysis prescribed by the Health Canada and/or United States Department of Agriculture – Food Safety and Inspection Service (USDA-FSIS) or with approved alternative methodology.
Laboratories may refer to the Health Canada's Compendium of Analytical Methods and to FSIS - Microbiology Laboratory Guidebook websites to ensure that the most current versions are used.
Laboratories are responsible for:
- maintaining laboratory accreditation with the SCC or the CALA under the Standards "General requirements for the competence of testing and calibration laboratories" ISO/IEC 17025:2005 and "Requirements for the Accreditation of Agriculture Inputs, Food, Animal Health and Plant Protection Testing Laboratories (ref.CAN-P-1587 April 2008)
- demonstrating ongoing proficiency for requested testing through a microbiology proficiency testing scheme
- admitting and/or disposing of samples for testing according to the applicable criteria described
- conducting testing for according to the technical requirements in this guidance document
- indicating on the laboratory report specific Health Canada, USDA-FSIS or the approved equivalent method used
- maintain records of tests performed
5. Analytical methods
Use and adhere to methods that have published PVC. Where methods have no published PVC, develop your own PVC for your establishment and recalculate them periodically as process improves.
Samples must be analyzed using 1 of the E. coli (Biotype I) quantitation methods found in the official methods of Analysis of the Association of Official Analytical Chemists (AOAC), International, 16th edition, or by any method which is validated by a scientific body in collaborative trials against the 3 tube Most Probable Number (MPN) method and agreeing with the 95% upper and lower confidence limits of the appropriate MPN index.
Suggested E. coli quantitation schemes
If a generic 1 ml plating technique is used for E. coli quantitation for turkeys, geese carcass sponging sample analysis, the plate count needs to be divided by 4 to equal the count per cm2 of carcass surface area, such as 100 ÷ 25 = 4.
Similar conversions are not necessary for quantitation of E. coli/ml of poultry rinse fluid samples. To cover the marginal and unacceptable range for E. coli levels, the undiluted sample extract, as well as 1:10, 1:100, 1:1,000 and 1:10,000 dilutions should be plated, preferably in duplicate. Higher or lower dilutions may need to be plated based on the specific product.
If a hydrophobic grid membrane filtration method were used, the only difference is that filtrations shall be performed on 1 ml of the undiluted sample extract, as well as of 1:10, 1:100, 1:1,000 and 1:10,000 dilutions.
Additional dilutions of the original extract may need to be used if a 3 tube Most Probable Number (MPN) protocol is used. The 3 highest dilutions that were positive for E. coli are to be used to calculate the MPN. For turkey and geese samples collected by swabbing technique, MPN values f needs to be divided by 4 to obtain the count per cm2 of carcass surface area.
Laboratories must report the exact E. coli count. Where values are quantified below 1 cfu/cm2, the exact count should be reported such as 0.01 cfu/cm2, 0 shall not be reported as a test result.
AOAC has approved the following methods for generic E. coli quantitation in foods:
- 3-tube MPN method - AOAC 17.2.01 - 17.2.02;
- Modified 3-tube MPN method - AOAC 17.3.07 - Substrate Supporting Disc Method (ColiComplete®).
- Modified 3-tube MPN method - AOAC 17.4.01 - Fluorogenic Assay for Glucuronidase, Lauryl sulfate tryptose broth with added 4-methylumbelliferyl-β-D-glucuronide (MUG) is used in a 3-tube MPN method.
- Plating Method - AOAC 17.3.04 - Dry Rehydratable Film (Petrifilm E. coli Count Plate) Method
Note: use in lieu of plating only - Not for use as a carcass direct contact medium: see AOAC method for details.
- Filtration /Plating Method - AOAC 17.3.09 - Hydrophobic Grid Membrane Filter/MUG (ISO-GRID) Method