The Feeds Regulations, 2024 were published on July 3, 2024, and have replaced the Feeds Regulations, 1983. We are working to update this page and the remaining website to reflect the Feeds Regulations, 2024. As new updates and guidance continue to be released, please refer to the Feed regulatory modernization page for more information.
On this page
- Purpose
- Scope
- Introduction
- Regulatory authority
- Industry's role
- CFIA oversight
- Summary of steps taken when Salmonella is detected
Purpose
The purpose of this guidance document is two-fold. The first is to describe the role of industry in preventing the entry and spread of Salmonella in livestock feeds manufactured, sold or imported into Canada. The second is to describe the role of the Canadian Food Inspection Agency (CFIA) in verifying that industry is meeting their regulatory obligations in this respect. Note that livestock feeds refer to both mixed feeds and single ingredient feeds.
This document is considered to be evergreen and is subject to future changes. Any modifications made to this document, following appropriate internal and external consultation, will be communicated through the CFIA's Email Notification Service.
Scope
This guidance document is applicable to livestock feeds intended for domestic livestock species as defined by the Feeds Act, namely
- horses
- cattle
- sheep
- goats
- swine
- foxes
- fish
- mink
- rabbits
- poultry
As defined by the Feeds Regulations, poultry means
- chickens
- turkeys
- ducks
- geese
Introduction
Salmonella is one of the most common foodborne zoonotic pathogens causing health and economic impact in humans and animals. Salmonella is a
- major causative agent of human bacterial gastroenteritis and bacteremia, and
- a major cause of morbidity and mortality around the world
If steps are not taken during the manufacturing of livestock feeds, to mitigate the presence of, or contamination by, Salmonella, it has the potential to have a negative impact on the
- safety of the feed and food chain
- the health of animals and humans
While all Salmonella has the potential to cause illness and negative impacts, certain serotypes have been identified as posing a significant risk to livestock species and humans.
- Table 1 lists those serotypes that pose a high risk to both human and animal health (based on the frequency of incidence in human and animal clinical cases)
- Table 2 lists the high risk species specific serotypes that pose a significant concern to certain livestock species with little evidence that they pose a serious concern to humans
These lists are not exhaustive and may be subject to change. Note that, while the following tables provide additional information concerning certain serotypes, the detection of any serotype of Salmonella in livestock feed is a non-compliance.
Table 1: High risk serotypes
High risk serotypes
Salmonella Typhimurium
Salmonella Enteritidis
Salmonella Heidelberg
Salmonella Newport
Salmonella Thompson
Salmonella Hadar
Salmonella Infantis
Salmonella ssp I 4,[5],12:i
These serotypes pose a significant concern to both humans and animals, as they
- are not species specific
- can be hosted by animals (asymptomatic carriers)
- can cause infection or severe illness to animals and humans, and
- can contaminate foods of animal origin, resulting in a serious risk to humans
Table 2: Species specific serotypes of concern
Serotype
Species at risk
Salmonella pullorum
poultry
Salmonella gallinarum
poultry
Salmonella choleraesuis
swine
Salmonella abortusovis
sheep
Salmonella abortusequi
horse
Salmonella dublin
beef and dairy cattle
Salmonella arizonea
fish
These serotypes pose significant risk to specific livestock species. There has been little evidence to show that these serotypes pose a serious risk to humans.
Regulatory authority
Subject to section 3(3) of the Feeds Act, no person shall manufacture, sell, import or export in contravention of the regulations any feed that presents a risk of harm to human or animal health or the environment.
Establishments manufacturing livestock feeds are responsible for ensuring their products are safe and efficacious for their intended use, and labelled appropriately.
Note that currently, as described by section 3(a) of the Feeds Regulations, a feed for export from Canada and so labelled is exempt from the Feeds Act and regulations.
Industry's role
When a livestock feed is found to be contaminated with any serotype of Salmonella, and therefore is non-compliant, it is the responsibility of industry to react accordingly. This means that the contaminated lot must be handled appropriately to prevent the further spread of the contamination and the cause of the contamination should be investigated to prevent future contamination events.
- Preventive control plans
- Responding to the detection of Salmonella
- Options for contaminated products
Preventive control plans
A preventive control plan (PCP) or Hazard Analysis Critical Control Point (HACCP)-based program is a written document that describes how an establishment's hazards are identified and controlled as well as how other regulatory requirements (for example, labelling) will be achieved. PCPs are an anticipated regulatory requirement under the proposed Feeds Regulations, 2023. Although PCPs are not required under the current Feeds Regulations, the CFIA recognizes the value that PCPs (or HACCP-based programs) provide in supporting the production of safe food and feed products and promotes this approach.
With respect to Salmonella, a PCP will include:
- the outcomes from the establishment's hazard identification and analysis
- where Salmonella is identified as a hazard, a description of the control measures implemented to control Salmonella and evidence that the control measures are effective (for example, scientific literature, best practices, etc.), and
- procedures for verifying that the implementation of the PCP results in compliance (that is, no Salmonella detected)
For example, a facility Salmonella monitoring sampling program can demonstrate that PCP results in compliance
While establishments are not required to have an internal monitoring sampling program for Salmonella, the CFIA encourages this practice. Establishments are not obligated to notify the CFIA of the results of internal sampling. However, inspection staff do have the authority to request any documentation related to the manufacture of livestock feeds when conducting an inspection, including results from internal sampling monitoring sampling programs. The CFIA inspection staff may take the results of internal monitoring sampling programs into consideration when determining the scope of inspection activities to conduct (for example, sampling for Salmonella).
Responding to the detection of Salmonella
When Salmonella is detected in a lot, establishments are to respond in a manner that prevents the entry, or continued entry, of Salmonella into the feed and food chain. Note that the following describes situations in which the CFIA is not involved.
- Primary actions - product control and communication
- Risk assessment
- Secondary actions - product control and communication
- Root cause analysis
Primary actions - product control and communication
One of the first considerations is of the contaminated lot itself. Any affected product, whether it be a single ingredient feed or mixed feed, that is still under the control of the establishment should not be allowed to be distributed further domestically. Establishments are to handle the affected lot in a manner such as to prevent the potential spread of the contamination to other products and equipment within the establishment. Establishments may also choose to initiate communication with purchasers of the affected product at this point. The purpose of communicating at this point is to let purchasers know that a risk assessment will be conducted and that further distribution of the affected product should not occur.
Establishments are not required to notify the CFIA of internal sample results in which Salmonella was detected, though they may choose to do so and request guidance regarding appropriate product control measures.
Risk assessment
While primary product control actions are being taken, establishments should perform a risk assessment to determine the scope and impact of the Salmonella contamination event. The key parameters in determining the potential risk include:
- the serotype of the Salmonella (if known), with special consideration of those serotypes in table 1 and table 2
- the nature of the affected product (for example, if it is a feed ingredient versus a complete feed intended for a specific species of livestock), and
- the volume and distribution of the affected product (for example, if the end user is a livestock producer versus a commercial feed mill that would use the product for further manufacturing)
While serotyping takes time, it is a key parameter when determining the potential impact on both humans and livestock species. When the serotype is known, risk management decisions can be made in consideration of the information provided in tables 1 and 2.
Secondary actions - product control and communication
Based on the results of the risk assessment, further product control actions may be necessary for the affected product that is no longer under the control of the establishment. It is at this point that establishments would initiate their secondary product control procedures (for example, recall) and communicate the results of their risk assessment and their risk management recommendations to purchasers of the affected product, as applicable. The goal is eliminating or minimizing, to an acceptable level, any potential risks to livestock species or humans posed by the affected product that has not already been consumed.
Establishments are not required to notify the CFIA of any secondary product control actions that are taken. However, establishments are expected to have, and follow, written procedures indicating under what circumstances they would notify the CFIA. Additionally, inspection staff have the authority, when conducting an inspection, to request any documentation related to any product control actions that an establishment has taken.
Root cause analysis
Establishments should conduct a root cause analysis to determine the source of the Salmonella contamination. This will allow for a determination to be made if PCPs or other procedures need to be revised, if any equipment repair is needed, etc. to prevent a reoccurrence. This analysis can include a review of documents such as:
- receiving records
- maintenance records
- pest control product records
- monitoring records
- temperature records
Given the rapid movement of materials in the feed supply chain and the timelines associated with sampling and analysis, it is not uncommon that a contaminated lot will have been consumed. As such, actions to prevent recurrence of contamination are very important and the anticipated regulatory requirement for PCPs under the proposed Feeds Regulations, 2023 will allow the CFIA to place emphasis on this preventive aspect.
Options for contaminated products
Once the affected product has been controlled through primary and/or secondary product control actions, establishments can consider options for how to bring product from the affected lot into compliance with the Feeds Act and regulations. Note that the following points below describe situations in which the CFIA may, or may not, be involved.
Domestic options
- Re-processing at rendering establishments
- Use other than livestock feed
- Disposal
- Salmonella prevention and treatment products
- Downstream processing/use
Re-processing at rendering establishments
It is known that the typical parameters of rendering are sufficient to kill Salmonella. As such, it is recognized that contamination of product likely occurs post-processing. Rendering establishments may re-process product contaminated with any serotype of Salmonella as a means of bringing the product into compliance.
Rendering establishments using re-processing as an option will need to have, and follow, written procedures describing how further spread of the contamination within the establishment is mitigated. In addition, they will need to verify that the treatment is effective. For example, if a conveyance that is normally dedicated to transporting finished product is cross-utilized to transport product positive for Salmonella, appropriate cleaning and sanitization of the conveyance will be necessary before it is again used for transporting finished product.
Note, when a likely source of post-processing contamination has been identified, establishments can use sampling and testing as a means to verify and demonstrate that corrective actions have been effective.
If the affected product is under CFIA detention, prior authorization to move the contaminated product must be provided by the CFIA inspection staff. In this case, the rendering establishment must also provide inspection staff with information concerning how the product will be handled prior to the re-processing activity.
Use other than livestock feed
Products that are no longer intended for use in livestock feeds are no longer subject to the Feeds Act and regulations. Establishments will need to meet any legislation (that is, municipal, provincial, federal, including environmental regulations) applicable to a use other than livestock feed. Note that if a lot under CFIA detention is to be diverted to an alternate product stream, the establishment must provide the CFIA inspection staff with sufficient information to verify the change in product stream prior to the release of the lot from detention. The establishment should consult with the CFIA inspection staff to determine what information is sufficient in their particular situation.
Disposal
Disposal of the affected lot is to be done in a manner that does not allow access for livestock and in accordance with any other applicable legislation (that is, municipal, provincial, federal, including environmental regulations). Note that if a product under CFIA detention is to be disposed of, the establishment must provide the CFIA inspection staff with sufficient information to verify the disposal plan prior to the release of the product from detention. The establishment should consult with the CFIA inspection staff to determine what information is sufficient in their particular situation.
Salmonella prevention and treatment products
There are currently no products approved in Canada for the prevention or treatment of Salmonella contamination in livestock feed. These products are sometimes referred to as mitigants.
Downstream processing/use
Livestock feeds are required to be compliant before they are offered for domestic sale and/or distribution. There is currently no option to allow feeds contaminated with Salmonella to be sold and/or distributed to other facilities in Canada for:
- further processing.
- use as feed for a species of livestock not sensitive to the serotype present
Export options
Export of feed
As described by section 3(a) of the Feeds Regulations, a feed destined for export from Canada and so labelled is exempt from the Feeds Act and regulations. Note that if a product under CFIA detention is to be exported, the establishment must make a request to the CFIA inspection staff to be permitted to relabel the product as necessary to indicate it is for export. Note that the presence of Salmonella makes a product ineligible for documentation to support market access for livestock feeds issued by the CFIA.
Note that all other CFIA export requirements, such as those related to the export of animal by-products, still need to be met and exporters must always meet the requirements of the importing country.
Salmonella prevention and treatment products for feeds to be exported
Establishments that decide to use any Salmonella prevention or treatment products in livestock feeds intended for export must do so in a manner that does not result in cross-contamination of livestock feeds intended for domestic use with the prevention or treatment product. Note that the use of any Salmonella prevention or treatment products in livestock feeds will make that feed ineligible for documentation to support market access issued by the CFIA's Feed Program.
Establishments using such products will need to have, and follow, written procedures describing how cross-contamination of livestock feeds intended for domestic use with the prevention or treatment product is prevented. These procedures will likely include preventive controls such as segregation of treated product and flushing and cleaning of equipment using a validated method.
Note that other CFIA export requirements, such as those related to the export of animal by-products, always need to be met.
CFIA oversight
To verify that livestock feeds comply with the regulatory requirements of the Feeds Act and regulations, CFIA inspection staff regularly inspect establishments that manufacture, sell, or distribute livestock feeds and monitor products via product inspection activities (for example, sampling programs).
The CFIA carries out 2 Salmonella feed sampling programs:
- the "Salmonella monitoring sampling program" which randomly samples livestock feeds from commercial feed mills, rendering establishments, and single ingredient feed manufacturers; and
- the "Salmonella directed sampling program" which is a sampling program focused on establishments that have an unacceptable compliance history with respect to Salmonella or have tested positive for a Salmonella serotype that poses a high risk to humans and animals (table 1)
If Salmonella is detected in feed samples collected, regardless of serotype, the sampled lot is considered non-compliant and a response by the regulated party is required (for example, development and implementation of a corrective action plan). In addition to verifying that industry is meeting their regulatory obligations with respect to Salmonella, the monitoring sampling program provides a snapshot of random feed products through the year to monitor livestock feeds for the presence of Salmonella.
The CFIA may also conduct follow up activities if Salmonella detected in a feed sample collected by CFIA is considered to be linked to other feeds, foods, animals, or people who were ill with Salmonella.
- Salmonella monitoring sampling program
- Salmonella directed sampling program
- Salmonella positive feed samples linked to Salmonella detection in other products
Salmonella monitoring sampling program
- Types of livestock feeds to be sampled
- Collecting samples
- Handling of samples
- Assessment of samples
- Compliance and enforcement actions the CFIA may take
Types of livestock feeds to be sampled
Although most types of feeds can exhibit Salmonella contamination, data from monitoring programs and information from literature show that certain feed types have a higher prevalence of Salmonella contamination. These feed types include:
- rendered meals
- oilseed meals
- grains
- recycled food products, and
- mash feeds
While monitoring for hazards in all feed types is important, these higher prevalence feeds are preferentially sampled by CFIA inspection staff. Inspection staff may also use the compliance history of individual feed establishments to identify the types of feed that should be targeted as part of a monitoring sampling program.
Collecting samples
Monitoring samples are, with few exceptions, to be collected at the manufacturer of the targeted feed product. For example:
- rendered products are to be sampled at the rendering establishments that manufactured them
- feeds are to be sampled at the commercial feed mills that manufactured them
- oilseed meals are to be sampled at the oilseed processing establishments that manufactured them
Exceptions to this guidance, considered on a case-by-case basis, would be:
- in response to complaints from livestock producers where intact packages of feed are available for sampling
- receipt of information from local, national or international bodies identifying a possible concern or contamination event in a specific feed
- sampling imported feed products
In addition to collecting samples at the manufacturer of the targeted feed product, whenever possible feed products that are ready for distribution are to be sampled. Note that inspection staff can only take samples when and where it is safe to do so. Manufacturers are required to provide inspection staff all the necessary assistance in order for them to carry out their inspection activities and, as such, are expected to work with inspection staff so that samples can be collected in a safe manner.
Handling of samples
The goal of CFIA inspection staff is to collect samples that are representative of the status of the feed product at the time of sampling. For the Salmonella monitoring sampling program, samples are taken using sterile (aseptic) technique and in a manner such that they are representative of the entire lot being tested. This means that inspection staff will take a minimum of 10 sub-samples from different locations within the lot being sampled. The sub-samples will then be combined to form a composite sample for analysis.
Note that lot size is not defined by the Feeds Act or Feeds Regulations. The manufacturer decides what a lot is. It could be 1 day's production, a shift's production or even a discreet defined time frame or amount (for example, a tote). The manufacturer must be able to demonstrate how lots are defined and provide evidence they are applied consistently in the manner described. Manufacturers must also be able to demonstrate how different lots of product are kept distinct and separate. Note that manufacturers are required to meet the Health of Animals Regulations requirements related to the assigning of lots for those feed products that are impacted by the enhanced feed ban.
Occasionally, an inspector may sample from 2 (or more) different lots. In such cases, the inspector will treat them as separate lots and identify that they are separate samples.
Samples are kept cool as warm temperatures promote changes in microbial levels. All samples collected under this program are stored and shipped in properly sealed and insulated containers (for example, styrofoam coolers) with ice packs. Proper sealing of containers is critical to avoid changes to contaminant levels or moisture.
Samples are to arrive at the CFIA laboratory within 72 hours of collection. Samples that do not meet the laboratory's requirements for reception, including the 72 hour deadline for Salmonella samples, are considered unfit and disposed of.
Assessment of samples
All feed samples collected by the CFIA inspection staff for the purposes of the Salmonella monitoring sampling program are assessed in a 2-step process.
The first step is to determine if Salmonella is "not detected" or "detected" in a feed sample. This analysis is conducted at a CFIA laboratory using a qualitative method. The service standard for this analysis is 10 business days from the time of reception by the laboratory, though results are typically available in a shorter timeframe. If a feed sample result indicates that Salmonella is "not detected" the sampled product lot would be considered compliant with the Feeds Act and regulations and no further actions are required. If a feed sample result indicates that Salmonella is "detected" the sampled product lot would be considered non-compliant with the Feeds Act and regulations and follow up actions by inspection staff and the establishment would be necessary.
When Salmonella has been detected, the second step of the process occurs, which is to identify the serotype through whole genome sequencing. The service standard for this analysis is 20 business days from the initial detection of Salmonella, though results are typically available in a shorter timeframe.
Compliance and enforcement actions the CFIA may take
The CFIA's Compliance and Enforcement Policy is based on the concept of a compliance and enforcement continuum, which includes providing information, the assessment of compliance as well as responding to the non-compliance. This is done through a wide variety of approaches, including:
- communicating with regulated parties
- conducting inspection activities, and
- taking appropriate enforcement actions in responding to cases of non-compliance
As mentioned above, if a Salmonella sample result comes back from the laboratory as "not detected", then the CFIA inspection staff will notify the establishment of the result, and no further actions are required. If the sample results from the laboratory are listed as "detected", then the sampled feed is considered non-compliant. The CFIA's oversight of non-compliant feeds will focus on 2 different aspects; the handling of the non-compliant feed itself and preventing a reoccurrence of the same non-compliance.
Note that the CFIA only takes enforcement action on non-compliant sample results when the samples were collected by the CFIA inspectors and analyzed by the CFIA laboratories.
- Corrective action requests and plans
- Risk assessment
- Product control actions and recall
- Other potential enforcement actions
Corrective action requests and plans
After the initial detection of the Salmonella, while waiting for the serotype assessment to be completed, a Corrective Action Request (CAR) will be issued by CFIA inspection staff to the establishment. Establishments can request a CAR review if they disagree with a CAR or request a CAR extension if they require more time to respond. The establishment is then expected to develop, submit, and implement an effective Corrective Action Plan (CAP).
As part of the CAP, inspection staff will be looking for information that identifies what, if any, immediate product control actions were taken. For example:
- all affected product has been held
- distribution of product identified
- any unfed product recalled
- receiving establishments have been notified
The establishment will need to identify how the proposed plan will be verified for effectiveness, as well as ensure that it is implemented within the given timelines. Any CAPs that involve long term or complex construction changes are required to have an interim plan in order to show how the affected product will be produced in compliance with the Feeds Act and regulations until such time as the construction changes are completed. The CAP should also identify what actions the establishment will take with the affected lot of product (as described in Options for contaminated products). This plan is then submitted to CFIA inspection staff to verify that it is acceptable. Note that the determination by the CFIA that a CAP is acceptable only indicates that it contains the elements of an appropriate CAP. It is the responsibility of the establishment to verify that the CAP has been effective. If new information comes to light, for example serotype identification or the results of the CFIA risk assessment, the CAP may need to be revised and sent to the CFIA inspection staff for another review.
CFIA inspection staff will plan a follow up inspection within 30 calendar days of when the corrective measures were to be implemented. During this inspection, the inspection staff will review any associated records, as well as conducting an onsite inspection to ensure that the plan has been implemented effectively. CFIA inspection staff may take a monitoring sample at a future date to verify that the establishment is meeting their regulatory obligations with respect to Salmonella.
Risk assessment
As mentioned previously, the CFIA conducts serotype identification for all positive sample results. This information is used by the CFIA to complete a risk assessment for all livestock species that may have been exposed to the identified serotype of Salmonella. The serotypes identified in table 1 and table 2 will be of particular interest in this regard. In addition, consideration is given to the processing steps to which the affected product has been subjected. This risk assessment will determine if a recall of the affected lot to the livestock producer level is warranted, and if there is a need to alert any other CFIA programs (for example, food program) due to potential exposure of livestock to specific serotypes of Salmonella.
Product control actions and recall
Upon receiving a positive result for Salmonella in a sample, CFIA inspection staff have the authority to place any and all remaining product from the sampled lot under seizure and detention. They can also require that the establishment disclose the distribution of the affected product to facilitate additional follow-up, if required. Furthermore, CFIA inspection staff will verify that the establishment has followed their existing recall procedures, if applicable, for any of the non-compliant product that has been further distributed.
Other potential enforcement actions
If compliance is not achieved through the corrective action process, further applicable enforcement actions, as described in the CFIA Compliance Continuum, may be taken.
Salmonella directed sampling program
The compliance history rating of an establishment is determined by looking at the results for the Salmonella samples collected by the CFIA over a period of 5 consecutive samples (regardless of the product type or production line within the establishment from which the samples were collected). If feed samples have been assessed as positive for Salmonella in 3 out of 5 consecutive samples, the CFIA would consider the establishment to have an unacceptable compliance rating history. Conversely, if feed samples have been assessed as positive for 2 or less, out of 5 consecutive samples, the CFIA would consider the establishment to have an acceptable compliance rating history. If the establishment has an acceptable compliance rating history and has not had a serotype listed in table 1 identified in feed samples, then these establishments would remain on the monitoring sampling program.
If an establishment has an unacceptable compliance history as defined above, and/or high risk serotypes have been identified in the feed samples as listed in table 1, then they will be placed on the directed sampling program. The directed sampling program is designed such that a greater number of samples are to be taken by CFIA inspectors during a given timeframe. The duration that an establishment is on the directed sampling program is dependent on their compliance history, both prior to and during the directed sampling period. In general, 3 consecutive negative feed sample results under this program, over a minimum of 3 months and a maximum of 6 months, would be considered acceptable for removal of the establishment from the directed sampling program. The CFIA inspection staff will notify an establishment when they are no longer on the directed sampling program. If compliance is not achieved within the given timeframe, further applicable enforcement actions, as described in the CFIA Compliance Continuum, may be taken.
Note that the samples collected while a facility is on the directed sampling program do not contribute to the establishment's compliance rating history. Once an establishment has successfully implemented controls such that it is no longer on the directed sampling program, future samples taken by the CFIA will resume under the monitoring sampling program and the establishment's compliance history with respect to Salmonella would restart at this point.
The principles presented in monitoring sampling program section also apply to the directed sampling program.
Salmonella positive feed samples linked to Salmonella detection in other products
When Salmonella has been detected in a feed sample, the sample is subject to further analysis using whole genome sequencing (WGS). This technique generates a genetic fingerprint, which can then be compared to the genetic fingerprints from Salmonella samples collected from other feeds, foods, animals, or people who were ill with Salmonella. This comparison can be used to identify links between Salmonella detections.
Should a sample collected by CFIA be considered linked to Salmonella detection in a different product due to a match through genome sequencing, in addition to the monitoring and directed sampling activities described in this document, the CFIA may work with other public health partners, such as the Public Health Agency of Canada, to further investigate this link. Follow-up activities may be conducted to better understand the circumstances and to assist with identifying a possible source of contamination. Identifying the source of the Salmonella is critical in preventing the continuation or reoccurrence of contamination. In these events, the CFIA may also conduct additional sampling and request information to determine the movement and/or storage of products associated with the positive sample.
Summary of steps taken when Salmonella is detected
Note that these steps are the same whether Salmonella is detected under the CFIA's Salmonella monitoring sampling program or directed sampling program.
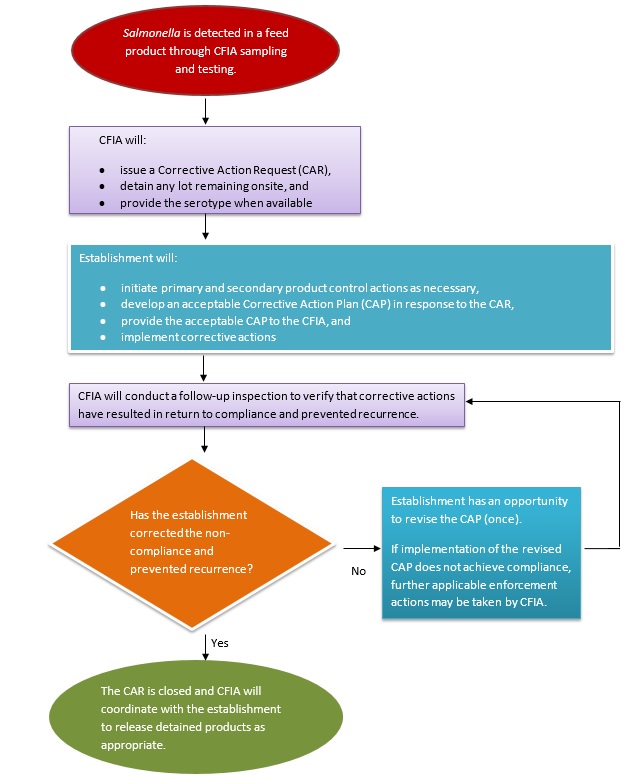
Description for photo - Summary of steps taken when Salmonella is detected
When Salmonella is detected in a feed product through CFIA sampling and testing:
- The CFIA will:
- issue a Corrective Action Request (CAR)
- detain any lot remaining onsite, and
- provide the serotype when available
- The establishment will:
- initiate primary and secondary product control actions as necessary,
- develop an acceptable Corrective Action Plan (CAP) in response to the CAR
- provide the acceptable CAP to the CFIA for review, and
- implement corrective actions
- The CFIA will conduct a follow-up inspection to verify that corrective actions have resulted in return to compliance and prevented recurrence
- If the establishment has not effectively corrected the non-compliance and prevented recurrence, they have an opportunity to revise the CAP (once)
If implementation of the revised CAP does not achieve compliance, further applicable enforcement actions may be taken by the CFIA - If the establishment has effectively corrected the non-compliance and prevented recurrence, the CAR is closed and the CFIA will coordinate with the establishment to release detained products as appropriate