On this page
- 1.0 Purpose
- 2.0 Overview
- 3.0 Acronyms
- 4.0 Glossary
- 5.0 Operational guideline
- 6.0 References
- Appendix 1: Vehicle biosecurity
- Appendix 2: Chemical disinfection
- Appendix 3: Biosecurity equipment and supplies
1.0 Purpose
This operational guideline outlines the biosecurity principles and describes the biosecurity measures that are required in the delivery of CFIA inspection activities within all 3 business lines (animal health, plant health and food safety). This document is:
- a foundational document that provides a framework for the Inspectorate to make decisions with respect to biosecurity measures
- a reference document for inspectors to support the incorporation of biosecurity concepts and measures into daily inspection activities
2.0 Overview
Biosecurity is a set of practices used to minimize the introduction and spread of hazards. Hazards can be transferred through many means including footwear, clothing, equipment and vehicles. A biosecurity breach can have devastating impacts on health, food safety and the environment which often results in significant financial losses. Everyone has a role to play in biosecurity and it is vital that CFIA inspectors take measures to reduce the risk of being a vector of hazards while conducting inspection activities.
Biosecurity measures are divided into 3 categories (exclusion, management and containment), based on their intent.
- exclusion prevents the introduction of hazards into a premises
- management minimizes the movement of hazards within a premises
- containment prevents the release of hazards outside of a premises
Diagram of the biosecurity categories
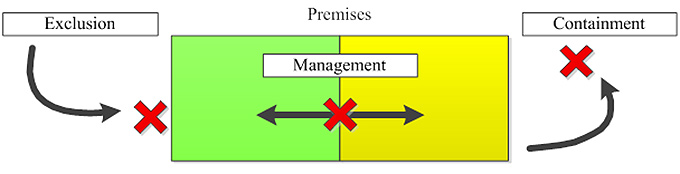
Figure 1: Diagram of the biosecurity categories
Exclusion limits the introduction of hazards into a premises. This is illustrated by an arrow that leads to a red X before the premises. Different areas within the premises are illustrated by a green and yellow rectangle. A double ended arrow crosses the coloured sections of the premises rectangle with a red X illustrating management of hazards within the premises. An arrow leads out of the premises rectangle to a red X illustrating the containment of hazards from the outside environment.
Therefore, biosecurity measures are to be taken by CFIA staff:
- before entering a premises to prevent the introduction of hazards (exclusion)
- while moving from one area to another within a premises to minimize the spread of hazards (management)
- when exiting a premises to prevent the spread of hazards to other premises (containment)
3.0 Acronyms
- CFIA
-
Canadian Food Inspection Agency
- DIN
-
Drug Identification Number
- HACCP
-
Hazard Analysis Critical Control Point
- iAIM
-
Integrated Agency Inspection Model
- OFFSP
-
On Farm Food Safety Programs
- PMRA
-
Pest Management Regulatory Agency
- SDS
-
Safety Data Sheets
- pH
-
Power of Hydrogen or Potential Hydrogen
- PPE
-
Personal Protective Equipment
- SIP
-
Standard Inspection Procedure
4.0 Glossary
- Antiseptic:
-
Chemical compounds used on living tissue (for example: skin) to inactivate disease-causing organisms to a level determined to be safe.
- Biosecurity:
-
A set of practices used to minimize the transmission of pests, diseases and contaminants including their introduction (exclusion), spread within populations (management), and release (containment).
- Basic biosecurity (Level 1):
-
Low risk, no contact inspection activities that are limited to areas where there is a low likelihood of the presence of a hazard that may present a potential risk.
- Routine biosecurity (Level 2):
-
Applies to inspection activities where there is moderate risk of contact with potential hazards. This biosecurity level is considered the standard for day to day inspection activities.
- Enhanced biosecurity (Level 3):
-
High risk of transmission of regulated diseases that are not highly transmissible and unregulated diseases that may be highly transmissible. To be employed for situations requiring heightened biosecurity. This may include enhanced bioexclusion for visits to livestock and poultry facilities (specific pathogen-free, breeding facilities, and other sites when necessary), situations requiring heightened biocontainment (suspicion or confirmation of an unregulated disease that may be highly transmissible or regulated disease that are not highly transmissible), or other applicable situations requiring enhanced biosecurity.
- Biocontainment:
-
A set of measures and procedures implemented to prevent the spread and release of pests, diseases and contaminants from a site.
- Containment biosecurity (Level 4):
-
A level of biosecurity that requires the implementation of control functions when a regulated highly transmissible hazard or public health concern is suspected or has been identified.
- Compliance verification:
-
An evaluation of a regulated party's compliance with legislative requirements using a combination of inspection and audit techniques.
- Contaminant:
-
Any biological, physical, chemical agent or other substance that is present in a regulated commodity and that compromises human, animal or plant health or the environment.
- Control function:
-
A specific set of activities and actions undertaken in response to an incident indicating an unacceptable or possibly unacceptable risk to human, plant or animal health or the environment. It is a response function when risk is realized.
- Direct contact:
-
Direct contact transmission requires physical contact between animals or plants or food products and a susceptible animal, plant or person and the transfer of the hazard. Personal Protective Equipment (PPE) minimizes the risk of transmission through direct contact.
- Disinfectants:
-
Chemical compounds applied to inanimate (non-living) objects to destroy or irreversibly inactivate micro-organisms, including disease causing micro-organisms.
- Disinfection:
-
Refers to the application of a physical or chemical process at an application rate effective in inactivating disease-causing organisms and includes but is not limited to chemicals, heat, and radiation/ultraviolet light.
- Exclusion:
-
A set of measures and procedures implemented to minimize or help prevent the introduction of pests, diseases and contaminants onto a site.
- Hazard:
-
As indicated in the Integrated Agency Inspection Model (iAIM), a hazard can be biological, chemical or physical. They are defined in the iAIM as follows:
Biological - Any illness- or disease-causing pathogen, micro-organism, pest or vector that poses a danger to human, animal or plant health or the environment.
Chemical - A chemical substance that poses a danger to human, animal or plant health or the environment.
Physical - Any foreign material that is not normally found in a commodity and that poses a danger to human, animal or plant health or the environment.
- Indirect contact:
-
Indirect contact transmission refers to situations where a susceptible host (animal, plant or person) is exposed from contact with a contaminated surface.
Some examples of surfaces that may become contaminated include:
- equipment
- food
- packaging materials
- vehicles
- clothing/footwear
- soil, water or organic matter
- Management:
-
A set of measures and procedures implemented to minimize or help prevent the spread of pests, diseases and contaminants between individuals within a site.
- Monitoring:
-
The act of conducting a planned sequence of observations or measurements of control parameters to assess whether a control measure is under control.
- Pest:
-
Any species, strain or biotype of organism injurious to or impacting on human, animal or plant health or the environment (including prions).
- Pesticide:
-
Pesticides, as the name implies, are used to control pests. The term generally encompasses both insecticides and fungicides. Insecticides are used directly against chewing and sucking insects and their larvae, while fungicides are directed against fungi, mildew and bacterial infections.
- Premises:
-
A geographically defined place that includes lands, establishments, buildings and facilities (for example: greenhouses, corrals, assembly centre).
- Risk:
-
Includes health, environmental, economic, social and consumer protection concerns. Risk is the product of the probability of an adverse event and the severity of its impacts. Biological, chemical and physical hazards which threaten human, animal or plant health or the environment (including economic and social impacts) are considered in assessing risk.
- Sanitizer:
-
Chemical compounds that reduce but do not completely eliminate the number of organisms on inanimate objects.
- Vector:
-
A carrier, or an agent, capable of transmitting a hazard from an infected source to a host.
5.0 Operational guideline
5.1 Biosecurity principles
Many hazards can be transmitted readily among plant and animal populations or a food processing environment. CFIA inspectors need to keep in mind that they may serve as vectors for hazards and inadvertently transfer these hazards within and to other premises on clothing, footwear, equipment and/or vehicles during their activities.
A routine day may require an inspector to visit one or more premises. Depending on the situation, these premises may be operating under various biosecurity levels which could potentially increase the risk of introducing and spreading hazards to the next inspected premises. Therefore, the inspector must schedule their day to ensure they have adequate resources to implement biosecurity measures between inspection activities. When possible schedule inspections of the same biosecurity level on the same day when multiple inspections are required. When multiple inspection activities are required at premises of varying risk the inspector should plan inspections from the least risk of contact or transmission of a hazard to the highest risk. Similarly, inspectors must also plan their inspection activities in each premises to reduce the risk of spreading a hazard by moving from areas and activities of lower risk of contact or transmission of a hazard to the highest risk.
Note
Be aware that soil, bodily fluids (either directly from live animal contact or indirectly from the slaughter process) and various surfaces may harbour hazards that can inadvertently be transferred. It is important to ensure appropriate measures are effectively implemented to mitigate the potential risk.
Managing the risks associated with inspection activities is very challenging as hazards are not always easily identifiable, often are not visible and many can only be confirmed with testing. Given this reality, CFIA inspectors visiting premises must use a precautionary principle and assume hazards are present, be aware of the risk they pose for spreading them and must take appropriate precautions.
Many producers will have biosecurity plans in place, particularly those in sectors and commodities that have adopted a set of standardized food safety practices, for example: On Farm Food Safety Programs (OFFSP), to meet domestic and international commerce requirements. In the case of food processing, many facilities have Hazard Analysis Critical Control Point (HACCP) programs in place. Many of these programs will have biosecurity requirements. It is important that CFIA inspectors are aware of and comply with the specific biosecurity requirements of a premises. In some cases, a premises may have biosecurity protocols that are more stringent than the applicable CFIA biosecurity level and CFIA staff must comply with the higher level of biosecurity.
The CFIA has developed commodity specific guidance documents for producers to assist them in the development of their biosecurity plans, for example, for terrestrial animals (National Biosecurity Standards and Biosecurity Principles - Animals - Canadian Food Inspection Agency) and for plants (Crop Biosecurity - Plants - Canadian Food Inspection Agency). Commodity specific biosecurity information can be accessed through Merlin under its business line information.
5.2 Determining the required level of biosecurity
The level of biosecurity required for inspection activities is determined by the level of potential risk. As CFIA inspectors, it is necessary to be proactive and provide leadership in the prevention of transmission of hazards. The difficulty is that many hazards are not visible until the signs or symptoms are apparent and test confirmation of the hazard is acquired, which could take hours, days or weeks. Therefore, proactive biosecurity is based on the assumption that a hazard could be present.
The continuum of biosecurity includes proactive measures to minimize the risk of introducing a hazard, monitoring for hazards and containing hazards once they have been identified. The biosecurity effort required is directly related to the risk of the inspection activities and classified as levels, for example level 1 for low risk.
It is vital that inspectors are aware of the potential risks involved with day to day inspections and plan accordingly to mitigate the risks. The biosecurity measures required greatly depends on:
- the inspection activity
- the commodity involved and the type of operation
- target population
- the status (the degree of risk or hazards present) of the premises
- the potential and existing hazard(s) encountered during inspection activities and their potential impacts
- aggravating factors (for example: wind)
- the surrounding risk situation in the area
- the inspector's schedule
Considering this, the following questions need to be answered when preparing and scheduling inspection activities.
- Are there official CFIA programs requiring biosecurity measures?
- Does the premises have a biosecurity plan or other programs in place that require specific biosecurity measures?
- What level of biosecurity should be applied to the premises to be inspected?
- What do I need to do to prevent:
- the introduction of any hazards onto the premises during my inspection (exclusion)?
- the spread of hazards within a premises during my inspection (on-site management)?
- the release of hazards from a premises during my inspection (containment)?
- How should I plan and schedule my inspection(s) to mitigate the risk?
- What supplies do I need to implement the required biosecurity measures?
- Are supplies available at the premises?
Always validate the status (the degree of risk or hazards present) of a premises and surrounding area prior to conducting an inspection activity and review the history of concerns reported from previous inspection activities. Validation will require communication with the premises management prior to the visit to ensure new information is considered as it may change the biosecurity level required or postpone the visit.
This communication will also identify if additional biosecurity measures are required as part of the premises or facility biosecurity requirements. Some specific biosecurity requirements of a premises may include: sign in procedures, footwear covers, foot baths, over coats, hairnets that may be required to transit through an area to reach an office, a designated parking area, etc. Finally, the communication will help establish any protective personal equipment (PPE) that is supplied by the operator which assists the inspector to identify supplies that need to be brought to the site. Some examples of PPE include boots, boot covers, coveralls, over coats, gloves, masks, hairnets, etc.
Importance of PPE
PPE provides a barrier that protects people from direct and indirect contact with hazards. It also provides an effective mechanism to contain a hazard when PPE is removed properly and placed in sealable containers or disposed of on a contaminated site. The extent of PPE required is directly linked to the risk probability, that is, the nature of the hazard and the severity of its impact, and the level of biosecurity effort required for the inspection activity.
5.3 Biosecurity levels
Biosecurity measures are divided into 4 levels.
- Basic (Level 1)
- Routine (Level 2)
- Enhanced (Level 3)
- Containment (Level 4)
Each level represents a collection of biosecurity measures. Some specific inspection activities already require CFIA biosecurity protocols, therefore, CFIA inspectors must identify if official biosecurity requirements exist while planning their inspection activities.
It is recognized that not all inspection activities delivered by CFIA inspectors will be a "perfect" fit into one of the 4 levels, therefore, some risk determination and judgement may be necessary for an inspector to determine the most appropriate combination of precautionary measures.
Notes
- The diversity of inspection activities delivered by CFIA staff often will require some risk decision making to determine the most appropriate combination of precautionary measures within a level and sometimes across levels (for example: routine to enhanced biosecurity).
- Transmission of hazards can occur in various ways such as direct or indirect contact with an infected animal, plant, vector, food, surface or organic matter (fecal-oral, mucous, blood, soil, etc.). Transmission can also occur when a hazard becomes airborne (coughing, sneezing, dust particles or organisms in droplet nuclei). The type of biosecurity measures required to prevent the transmission of hazards will be dependent on various hazard characteristics. Refer to commodity specific plans for information on the types of transmission routes that should be considered for a particular hazard.
5.3.1 Basic biosecurity (Level 1)
Basic biosecurity applies to low risk, no contact inspection activities, that are limited to areas where there is a low likelihood of the presence of a hazard that may present a potential risk. No contact is anticipated with potentially hazardous material that could result in a contamination of the person, their clothing, footwear, equipment, and/or vehicles. Arriving clean and leaving clean reflects the very minimal biosecurity measures required and illustrates respect and professionalism.
Examples of work/inspection activities that may require Basic Biosecurity measures include but are not limited to:
- meetings or training in an office environment
- documentation reviews
- visits to a private residence or sales office
- office work at a CFIA location
Notes
- If the inspector needs to transit through a higher risk area to reach the inspection area, the level of biosecurity applied must account for the higher risk.
Best practice: Be prepared for the unexpected
Even the best planned day may bring an unexpected event that was not shared in the pre-visit communication, such as the identification of a hazard or an unexpected event occurs that elevates the potential risk when you are on-site. It could also be as simple as a regulated party requesting guidance which may result in contact with a production area that was not originally part of the planned inspection activity.
Assess the risk: options may include implementation of heightened biosecurity measures or delaying the visit. In either case, it is a good practice to have supplies and PPE available to elevate your biosecurity measures on-site or while exiting, should the "unexpected" occur.
Basic level biosecurity measures
Planning
- Contact the premises to discuss biosecurity processes/concerns and requirements
- Ensure clothing, footwear and any materials and equipment are clean (disinfect if necessary)
- Collect cleaning and disinfection supplies
- Store materials and equipment in a clean area of the vehicle
- Ensure the inside and outside of the vehicle are visibly clean
Inspection
- Wash and/or sanitize your hands prior to entering the premises, during transit within, if required by the premises and upon exiting
- Park in the designated visitors' area and avoid contact with manure, organic material and standing water
- Notify the operator of your arrival and sign the logbook if required
- Take only materials and equipment required for the work activity
- Do not enter any controlled, restricted or production areas (avoid potential biosecurity hazards)
- Clean and disinfect any equipment if necessary
Post Inspection
- If visibly soiled or if unintended contact with hazards occurred, change or contain clothes on returning to the office or before visiting another premises
- If the vehicle becomes grossly contaminated with organic material, wash it before visiting any other premises
- Restock supplies
To disinfect or not to disinfect - that is the question
Whether it is your footwear, the vehicle's tires, wheel wells or under carriage, below are few examples of situations that may require disinfection when performing a basic or routine level inspection activity.
- During preparation for the inspection you become aware that the area has a previous history of hazards. As a result, there is heightened awareness of the vulnerability of the commodity sector to hazards.
- The regulated party requires disinfection prior to arrival and upon exiting the premises. The regulated party may have the perception that government inspectors are responsible for spreading hazards. Implementing additional precautions may mitigate these concerns and improve working relationships.
- There is an emerging hazard of concern for Canada that may impact the capacity of the regulated party to maintain current markets and access new ones.
- You have inadvertently walked or driven through organic material, waste water, secretions, fluid and/or manure.
Lead by example to encourage the regulated party to implement best biosecurity practices.
5.3.2 Routine biosecurity (Level 2)
Routine biosecurity applies to inspection activities where there is moderate risk of contact with potential hazards. This biosecurity level is considered the standard for day to day inspection activities. Routine biosecurity precautionary measures are in addition to the minimum Basic Biosecurity measures described previously.
Implementation of precautionary biosecurity measures can mitigate the risks associated with these potential hazards. Biosecurity relies on the consistent application of routine measures. It begins prior to leaving home and continues after returning home from work. Staff who come into contact with hazards during their personal activities must ensure that these are not inadvertently transmitted during their work duties. Personal clothing and vehicles can become contaminated and transmit hazards.
The majority of CFIA inspection activities will require routine biosecurity precautions and will involve compliance verification and monitoring. The information provided by these activities may elevate the level of biosecurity required when conducting inspection activities at a premises. If the presence of a non-regulated or regulated hazard is suspected or identified, the biosecurity level may be elevated to Enhanced Biosecurity (Level 3) or containment (Level 4).
Examples of inspection activities that may require Routine Biosecurity measures include but are not limited to:
- vineyards
- orchards
- berry farms
- seed potato farms
- processing audits
- compliance verification of a preventative control plan
- slaughter/meat inspections
- field inspection
- biological surveys for pest detection
Routine level biosecurity measures
Adequate preparation and planning of the site visit are necessary to ensure that communication between CFIA staff and the owner/manager/producer occurs in order to minimize the risk of biosecurity lapses and the spread of hazards.
Planning
- Be aware that the premises may already have established biosecurity procedures. For premises where stricter biosecurity measures are implemented, inspectors must respect those measures
- Determine site-specific biosecurity procedures to be used for entry and exit
- Contact owner/producer/manager to schedule a date and time for the visit
- Determine current animal/plant health status. The presence or recent occurrence of a transmissible hazard at this or other sites owned by the individual increases the risk of disease transmission within and on/off the premises. Additional planning is necessary to ensure that the risks are addressed
- Try to schedule only 1 visit per similar facility per day. If this is not feasible, visit low risk/vulnerable premises before higher risk/less vulnerable premises
- Follow this procedure for multi-stage production sites
- Determine which biosecurity supplies (for example: coveralls, boots, and/or water) are provided (facility dedicated)
- Collect all equipment and supplies required for the site visit and make sure they are clean and stocked in the biosecurity kit
- Ensure the inside and outside of the vehicle are clean and store any materials/equipment properly
- Mix disinfectants as per manufacturer's instructions
Inspection
- Drive slowly onto the site
- Park your vehicle in the visitor or designated parking area or in an area which is clean and dry, and away from barns and production areas
- Take only materials and equipment required for the inspection activity
- Notify the producer of your arrival and comply with any additional biosecurity protocols
- Don clean PPE in a designated area. For example:
- parking area of primary plant and poultry/livestock production
- uncultivated area of a field
- designated changing area for primary and secondary food processing facility
- Ensure footwear is clean and disinfected before entering production areas
- Wash hands with a suitable sanitizer or disinfectant
- Sign the visitors' log if there is one
- Consider the following logistical flows when conducting inspection activities:
- from clean to dirty areas
- from youngest to oldest animals
- from propagation/mother-stock plants to production houses/fields
- from healthy to diseased or infested
- from low to high risk areas
Note
If an ideal logistical flow as identified above is not possible, then additional biosecurity efforts would be required to minimize the potential transmission of hazards.
- If required, don nitrile, latex, or rubber gloves
- Move along hard/clean surfaces, such as concrete or asphalt, where possible
- Clean and disinfect outerwear, gear, and equipment between different areas of the premises, when appropriate
Leaving a premises
Note
Many of the measures listed in the following sections begin with "If applicable…" or "Where possible…" These mentions are intended to allow for protocol differences for Routine Biosecurity within business lines or commodities.
- If applicable, place contaminated disposable equipment and garbage in a plastic garbage bag and leave it (with the owner's permission) at the premises for disposal
- If applicable, prior to leaving the premises, clean the exterior of all gear, containers, and equipment with a detergent solution and rinse to remove any organic debris
- If applicable, spray all gear, containers, and equipment with disinfectant, or immerse in a disinfectant solution. Leave the disinfectant on for the proper contact time, according to manufacturer's directions
- If possible, rinse gear, containers, and equipment with clean water after the disinfectant contact time has elapsed – this will be dependent on the disinfectant used
- If air drying is impossible, dry the gear, containers, and equipment with towels. Either discard the towels in the premises garbage (in a sealed bag), or place them in a plastic bag and seal. Spray the outside of the bags with disinfectant
- If disinfection is not possible at the facility, clean equipment of gross contamination, and place in a container or bag that can be sealed for removal from the site for cleaning and disinfection later. Wipe the outside of the bag/container with disinfectant prior to removal
- Clean and disinfect hands
- Follow any other premises-specific biosecurity procedures regarding exit that may be in place
- Check for any visible hazards or pests on clothing, equipment and vehicles
Vehicle processes
A detailed description of the vehicle cleaning and disinfection process can be found in Appendix 1.
Post inspection
- Remove gear, containers/totes, and equipment from the "clean" area of the vehicle
- Remove sealed bags or containers/totes from the "dirty" area of the vehicle
- Clean vehicle - see Appendix 1
- Clean and disinfect any gear, containers/totes, and equipment that could not be cleaned at the premises
- Appropriately dispose of gear, containers, and equipment that cannot be cleaned or disinfected
- Ensure clothing and footwear are clean before entering the office
- Launder dirty coveralls using biosecure procedures or a contracted laundry service
- Re-stock totes designated for clean items with clean materials and disinfected equipment
- If applicable, shower thoroughly and wash your hair
- Launder personal clothing worn on the premises
5.3.3 Enhanced biosecurity (Level 3)
The biosecurity level is elevated from basic and routine to enhanced biosecurity when:
- a higher risk has been identified (suspected or confirmed hazard) on a premises
- inspectors visit facilities with vulnerable animals or plants
- inspectors visit facilities producing specific products destined to a high risk population
Generally, the situations that could be encountered are:
- a suspected or confirmed non-regulated hazard that is highly transmissible
- a suspected or confirmed regulated hazard that is not highly transmissible
- a high risk population
Examples of inspection activities that may require Enhanced Biosecurity measures include but are not limited to:
- inspection activities related to import/export certification programs (plant inspection may require only level 1 or 2)
- laboratory inspections
- inspection of a pathogen free porcine facility
- auction market inspections (ID verification and humane transportation)
- hatchery inspections
- artificial insemination inspections
- plant production (greenhouses) that import plant stock intended for propagation
- nurseries
What is high risk population?
A high risk population is a population of animals, plants or people that are significantly more susceptible to hazards. Examples of these include:
- animals that are susceptible to certain pathogens
- animal collection hubs or auctions
- vulnerable animals or plants (propagative material)
- plants (such as nuclear stock) that have been bred and tested to be free of viruses or other disease agents
- people with sensitivities or allergies to food and/or food ingredients (for example: peanuts, eggs, or gluten)
- children, elderly, pregnant women and immunosuppressed individuals
- people with special dietary considerations
Enhanced biosecurity level measures
Note: Employ all measures in the Routine Biosecurity section, including additional measures as follows:
High risk populations
Planning
- Provide site management with a list of equipment and materials that you will bring onto the site. Some facilities may refuse entry of non-facility equipment. Arrange in advance the procedures to clean and disinfect equipment and supplies to their satisfaction
- Communicate with the premises the number of CFIA staff that will be attending and any pre-visit biosecurity measures
- Allow sufficient time for the site visit
- Showering in, showering out, and donning site-specific clothing may be required
Inspection
- Follow site-specific procedures
- Allow site managers to re-clean and disinfect equipment and materials or provide site-specific equipment and materials if requested
- Avoid unnecessary exit and re-entry to the site
- Be prepared, enter the site, complete the required tasks, and exit the site
- Respect requirements for accessing biosecurity zones, segregation, and isolation facilities, including nursery and breeding areas that may employ additional protocols
- Follow Routine Biosecurity measures for exiting the site
Post inspection
- Follow Routine Biosecurity measures upon arrival back at the office
Non regulated hazards – highly transmissible
Planning
- Obtain detailed information regarding the hazards at the facility
- Schedule your visit as the last activity of the day
- Request producer set-up boot washes (boot pail, clean disinfectant, and scrub brush) at critical points
- Gather additional biosecurity materials. Consult CFIA specialists, your supervisor, and Occupational Health and Safety if there is a risk to human health
- Prepare additional disinfectant as per manufacture's recommendations
- If required, take additional cleaning and decontamination supplies and equipment
- If required, take materials for posting any required biosecurity signage
Inspection
If required by a CFIA program:
- Establish an area for cleaning and disinfection of personnel and equipment
- Avoid unnecessary exit and re-entry to the site.
- Leave disposable materials/garbage on the site for disposal by owner
- If wearing gloves, remove one layer on site in a clean location prior to washing equipment.
- The second pair will be removed with the coveralls
- When removing coveralls, take additional care to prevent contamination of inner layers
- Take extra care when cleaning and disinfecting boots and equipment. If properly cleaned, re-pack in clean containers. If only partially cleaned, contain in a "dirty" tote for re-cleaning and disinfection off-site or before moving across areas with different biosecurity levels
To minimize disease carriage off-site, advise the producer to:
- seek private professional advice
- restrict the movement of animals, plants, products, people, materials, equipment, and things onto and off the site until the owner has received professional guidance
- post biosecurity/no entry signage at premises entrance (roadway)
Note
The primary responsibility for controlling and responding to unregulated disease occurrences resides with industry (producers and their associations) and the information is provided to producers as advice. Producers are not required to implement the recommendations provided by CFIA.
Regulated hazards – not highly transmissible
- Follow CFIA investigation protocols
- As a minimum, notify your Supervisor and Operational Specialists
- Staff attending premises with confirmed regulated hazards (not highly transmissible) shall not attend other premises without undertaking appropriate personal biosecurity measures
- Select an appropriate disinfectant based on the hazard. See information listed in Appendix 2: Chemical Disinfection
- Ensure that control actions for the facility (movement restrictions etc.) are appropriate for the hazard and have been issued to the producer
- Post biosecurity/no entry signage at the premises entrance (roadway)
- Advise the owner to restrict access to the site
- Clean and disinfect all equipment thoroughly before leaving site
5.3.4 Containment biosecurity (Level 4) – suspected or identified risk – regulated highly transmissible hazards
Containment biosecurity is the level of biosecurity that requires the implementation of control functions when a regulated highly transmissible hazard or public health concern is suspected or has been identified (for example: food recall, water advisory warning). Containment (level 4) biosecurity measures are well defined (for commodity specific information on biosecurity, inspectors consult with their respective subject matter experts using established communication channels) and are supported by a control function that provides for compliance and enforcement activities.
The CFIA has many types of control functions that are designed to mitigate the impact of a hazard. Some control functions that can be implemented are recall, disposition, quarantine, order to treat, prohibition of movement, declaration of a controlled area, confiscation, declaration of an infested or infected place, refused entry, refused certification for export, removal and public warning (a Range of Regulatory Actions can be found in the iAIM Annex D). The biosecurity activities to ensure containment of the hazard are determined by the risk posed to the inspector, as well as the nature of the hazard, the severity of its impact and the risk of spread.
Examples of inspection activities that may require containment biosecurity measures include but are not limited to:
- response to a highly transmissible reportable hazard
- response to a highly transmissible notifiable hazard
- other hazards monitored by the CFIA
- recalls
- potato cyst nematode sampling in regulated areas,
- plant pest containment facility
Note
If the presence of a highly transmissible regulated hazard is suspected or confirmed at a premises, strict controls will be implemented by the CFIA Incident Command Structure. Specific biocontainment, hazard response and surveillance measures will be in place. In this situation, routine inspection duties will be halted and access to the premises highly restricted and controlled.
Containment level biosecurity measures
If, while on a premises, staff suspect a highly transmissible regulated hazard, additional measures must be taken to prevent hazard carriage off-site and to ensure proper notification to CFIA staff.
- Follow CFIA investigation protocols
- Immediately notify your commodity specialist (subject matter expert) and your supervisor
- Review and follow specific recommendations as outlined in hazard specific plans if available
- Depending on the hazard suspected and the level of certainty, determine whether the owner of the premises should institute lockdown procedures
- Post biosecurity/no entry signage at the premises entrance (roadway)
- Advise the owner to restrict access to the site
- If allowed or instructed to leave the site, do a full decontamination of outer clothing (coveralls, boots), equipment and follow other routine or recommended biosecurity procedures
Figure 2: General biosecurity level decision tree
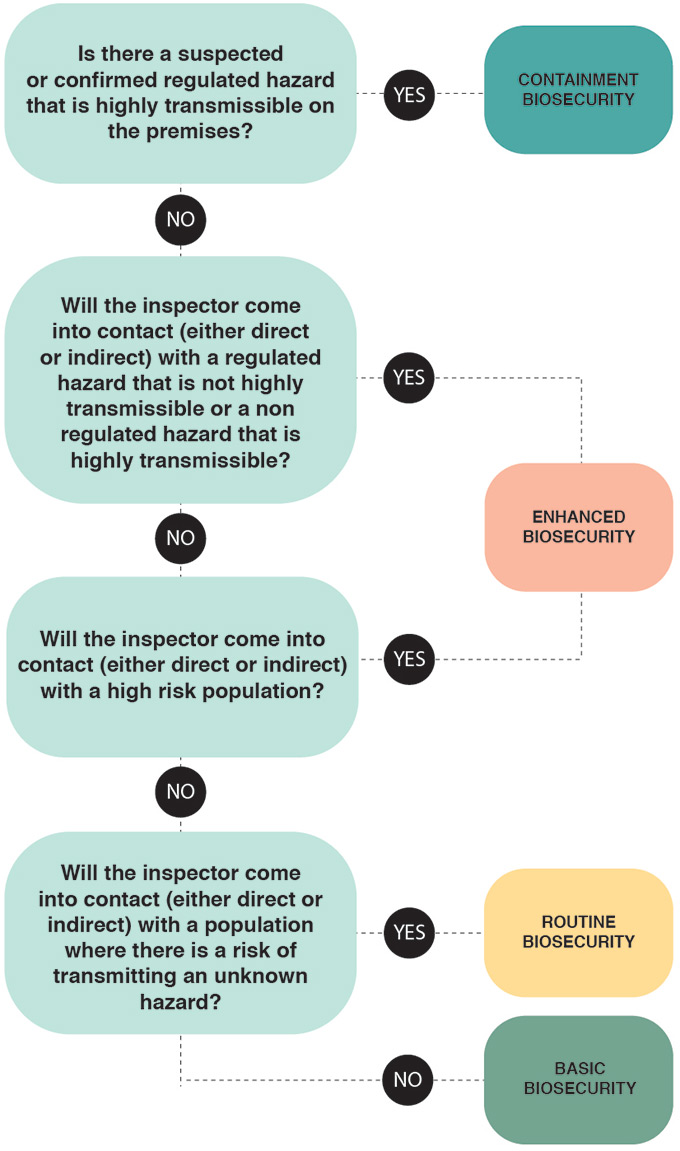
Figure 2: General biosecurity level decision tree
If there is a suspected or confirmed regulated hazard that is highly transmissible on the premises the inspector should use containment biosecurity measures. If not but the inspector will come into contact (either direct or indirect) with a regulated hazard that is not highly transmissible or a non-regulated hazard that is highly transmissible then enhanced biosecurity measures are to be used. If not but the inspector will come into contact (either direct or indirect) with a high risk population then enhanced biosecurity measures are still to be used. If not but the inspector will come into contact (direct or indirect) with a population where there is a risk of transmitting an unknown hazard then routine biosecurity measures are to be used. If not then basic biosecurity measure are to be followed.
6.0 References
- Biosecurity summary for inspectors (English version - internal access only)
- integrated Agency Inspection Model (iAIM)
- Standard Inspection Procedure (SIP)
For any questions or additional guidance on this document, follow the normal communication channels in the Area.
Appendix 1: Vehicle biosecurity
Note
The occupant area of any vehicle cannot contain loose objects. Any equipment or storage items in this area must be secured. If this is not possible the trunk or box of the vehicle must be used.
- Vehicles should be clean prior to site visits. Critical areas include the chassis, wheel wells, tires, equipment storage areas, seats, and floor mats (areas where organic material can accumulate and cross-contaminate objects such as footwear/equipment or physically be deposited on the site)
- Designate clean and dirty compartments in the vehicle
- For sedans, the clean compartment may be the passenger area and the dirty compartment the trunk
- In trucks with extended cabs, the clean compartment may consist of the area behind the front seats or the truck box may be divided into clean and dirty sides with a plastic or other suitable divider
- Separate and store clean and dirty items in totes. Ensure totes/plastic containers are designated and labelled as clean or dirty
- Store clean equipment/materials inside clean totes in plastic containers/bags ready for use. In the event that contamination of the tote occurs, materials will remain clean. The external surface of totes should be cleaned and disinfected after each site visit and as necessary; the inside surfaces should be cleaned and disinfected as necessary
- Store clean coveralls and forms in the clean compartment until used on the premises
- Never enter the clean compartment wearing used coveralls or place garbage bags and items that have been on the premises in the clean area. Always move equipment from the clean to the dirty compartment of the vehicle
- To facilitate cleaning and disinfection of the driver's seats, consider using a washable seat covering
- Provide rubber (washable) floor mats for each person in the vehicle
- Protect trunks and truck boxes with a single piece of rubber or heavy plastic liner, which can be removed for cleaning and disinfection
Remember never to enter the clean compartment with soiled footwear and/or clothing.
Note
The above procedures are intended for routine inspection duties. If the presence of a hazard is detected on the premises, intensive procedures specific to the situation must be followed.
Day-to-day procedures
Vehicles should be cleaned after premises visits. The degree of cleaning depends on the degree of contamination and degree of risk posed by the inspection activities. However, staff must assume a level of risk for all site premises visits according to the minimum cleaning described below.
Exterior of vehicle
The vehicle should be visibly clean with no accumulation of organic debris. Pay particular attention to the chassis, wheel wells and tires. If there are small accumulations of debris, cleaning with a stiff-handled brush and disinfection with a hand sprayer may be sufficient. If visibly dirty, or if staff has attended a site with suspect hazard, the vehicle should be thoroughly cleaned. (See below).
Inside of vehicle
Floor mats should be visibly clean. Using a disinfectant, spray or wipe down floor mats and steering wheels.
Trunks or truck beds
Ensure they are visibly clean and wipe down with disinfectant any areas where dirty equipment was placed. If visibly dirty, or if staff has attended a site with suspect hazard, the vehicle should be thoroughly cleaned. (see below).
Where a hazard is suspected and/or when vehicles are heavily contaminated:
Exterior of vehicle
- Wash vehicle at a commercial car wash or use a pressure washer or scrub brush and hose
- Use hot water and detergents
- Wash the exterior chassis surfaces, tires, wheel wells and rims, the step plates and any boot brush and access area, and if possible the undercarriage
- If the vehicle is a pickup truck or cube van, wash the box including the floor and sides and any external storage compartment(s)
- Apply disinfectant to all exposed surfaces if required (enhanced biosecurity and biocontainment conditions)
- Protect sensitive environmental areas from waste water (for example: commercial car wash
Interior of vehicle
- Remove rubber floor mats and clean with pressure washer. Disinfect and allow to dry
- Vacuum seats, floors and trunk, removing any bins or equipment
- Wipe the steering wheel with water and detergent, or a disinfectant wipe
- Brush or wipe down with water and detergent: the seat (or seat covering if there is one), pedals, door handle, control panel(s) and radio/telephone. Disinfect and allow to dry (disinfectant wipes or sprays or solutions). If the surfaces are visibly clean, dry and wet cleaning prior to disinfection may not be necessary
- Clean the garbage receptacle, and replace any bags, disposing of garbage
Appendix 2: Chemical disinfection
Product regulation
Health Canada regulates the registration of disinfectants and pesticides under the Pest Management Regulatory Agency PMRA) in Canada and provides a Drug Identification Number (DIN) for disinfectants and a registration number for pesticides prior to their marketing. This DIN and registration number is listed on the disinfectant container.
Selecting a disinfectant or a pesticide
Disinfectants and pesticides are evaluated by Health Canada using strict criteria. However, efficacy is determined under controlled laboratory conditions and if using disinfectants on a premises, they must be used according to the manufacturer's recommendations. Disinfectants and pesticides selection is based on a variety of factors, including the:
- chemical properties of the disinfectant
- type(s) of organism targeted
- cleanliness of materials to be disinfected
- composition (wood, metal, rubber, etc.) of surface to be disinfected
- temperature of surfaces and disinfectant
- contact time
- concentration
- application method/safety
- presence or use of other chemicals
- pH
- characteristics of water (presence of dissolved solids, degree of contamination)
- environmental considerations (presence of streams, wildlife)
- cost
These factors (in addition to others) will affect the ability of a disinfectant to perform as indicated by the manufacturer. Choose broad-spectrum registered disinfectants (those with a DIN) with minimal toxicity, that are easy to apply and effective under a variety of environmental conditions.
Chemical disinfectants
The following information is provided as a guide for staff when selecting disinfectants. Staff should refer to the Safety Data Sheets (SDS) and manufacturers' labels when selecting and applying disinfectants.
Chemical disinfectants are classified into broad categories based on their chemical structure. Each class of disinfectant confers different disinfectant properties and selection should be based on the criteria outlined above.
Summary table describing disinfectant class, efficacy and limitations (English internal access only).
Disinfectant storage
Chemical composition, physical state (dry, aqueous), method of packaging, and conditions of storage—these all affect the shelf life of a disinfectant. A few disinfectant classes (phenols and quaternary ammonia compounds) are very stable products and may be useful for offices where disinfectant turnover is minimal.
Many disinfectant have a "best-before date" which is based on proper storage and handling of the product. Chemicals degrade over time, reducing the effectiveness of the product; degradation often increases significantly after a product has been opened. Use unexpired disinfectants and ensure lids/tops/bags are securely fastened for storage. If there are manufacturer's recommendations on storage, follow them. If there are no recommendations, store in a cool, dry, dark place.
Disinfectant application
Disinfectants are most effective when applied to clean dry surfaces. Organic material (litter, soil, manure, etc.) on equipment, boots, and structures significantly reduce the activity of disinfectants. These surfaces must be cleaned (dry and wet cleaning) prior to disinfectant application. The following application steps will help improve the efficacy of disinfectants:
- physically remove all visible debris from the surface of the area to be disinfected
- wash and scrub all surfaces with hot soapy (when possible)
- rinse
- apply the disinfectant as per manufacture's recommendations
- allow for the required contact time
Follow the manufacturer's recommendations for application paying strict attention to the concentration required and contact time. Use warm to hot water when mixing and applying disinfectants (most disinfectants, detergents and soaps have increased activity in warm water). Some disinfectants require rinsing as their final step. The final step of the disinfection process is to allow all surfaces to dry.
Follow local government regulations regarding the application of disinfectants to ensure compliance with environmental legislation.
Once disinfectants are mixed with water or other chemicals, their shelf life decreases dramatically and disinfectant must be replenished regularly. This may be daily for some products and weekly for others.
Disinfectants used for cleaning boots and other heavily contaminated equipment must be replenished frequently and are only effective if properly applied; boot baths/dips when heavily used may be ineffective, and must be used with caution.
Appendix 3: Biosecurity equipment and supplies
The following list is a guideline for common biosecurity equipment and supplies. It is not comprehensive. Different situations will require specific equipment and supplies. For example a tote for storing contaminated equipment may not be necessary for a Level 1 Basic Biosecurity visit but disinfectant and a method of application should be part of a biosecurity kit for all inspection activities.
- Clean clothes and foot wear
- Clean vehicle (Appendix 1)
- Appropriate disinfectant (Appendix 2)
- Appropriate detergent or cleaner
- Disinfectant applicator, for example: spray-pack
- Disposable gloves
- Antiseptic hand gel/wipes
- Large clear plastic bags (for "dirty" equipment, clothing, and supplies)
- Proper disposal bags, such as autoclave bags or thick garbage bags
- Small plastic or wire brush (for cleaning instruments)
- Long-handled boot brush
- Disinfectant waste bucket (sealable)
- 5 gallon boot and gear bucket
- 4 litres of fresh water (if required)
- PPE as required