National Microbiological Baseline Study in Broiler Chicken
December 2012 – December 2013
A study conducted under the federal, provincial and territorial Pathogen Reduction Initiative in meat and poultry
May 12, 2016
Acknowledgements
The Canadian Food Inspection Agency (CFIA) would like to thank and recognize the substantial work carried out by its partners and the contributions of various organizations that made this national microbiological baseline study (MBS) in broiler chicken possible:
- Agriculture and Agri-Food Canada for providing the necessary operational funds to deliver the MBS including preparation, coordination, sampling and testing of poultry samples and communications.
- The Canadian Poultry and Egg Processors Council and its technical committee for providing technical support and coordination of the design and implementation of the MBS.
- The industry personnel and CFIA inspection staff at each federally-registered poultry establishment enrolled in the MBS for collecting broiler chicken and raw chicken meat samples.
- The provincial inspectors from the Regional Health Authorities, Public Health Units and Agriculture Ministries and CFIA inspectors from Waterloo region who collected raw chicken meat products from retail stores across the country for the MBS.
- The Laboratory for Foodborne Zoonoses of the Public Health Agency of Canada (PHAC) and the Fallowfield Laboratory of the CFIA for characterizing the bacterial isolates recovered from the positive samples.
- The Bureau of Microbial Hazards of Health Canada for their support in assessing the performance of the analytical methods during the course of the study.
- The Laboratory Services Division of the University of Guelph under contract for testing all broiler chicken and raw chicken meat samples during the pilot and study phases.
Contributors to MBS 2012-13
The CFIA would like to thank and recognize those individuals and organizations who participated in the analysis and reporting of MBS results.
Program Lead
Anne-Marie St-Laurent
Project Manager
Daniel Leclair
Project Coordinators
Lyne Brosseau
Jane MacDonald
Gary Thiessen
Laboratory coordination
Stan Gagnon
Daniel Leclair
Steve Lenz
Data validation and database management
Solomon Aklilu
Jean-Robert Bisaillon
Lyne Brosseau
Ryan Currie
Data analysis and reporting
Jean-Robert Bisaillon
Ryan Currie
Daniel Leclair
Alexandre Leroux
Reviewers
CFIA
Sujinder Bhachoo
Lyne Brosseau
Catherine Carrillo
Sukhpal Deol
Kate Hardie
Jane MacDonald
Gary Thiessen
FPT Pathogen Reduction Working Group
Valerie Bohaychuk
Jeanine Boulter-Bitzer
Jeff Farber
Denise Oudit
Leanne DeWinter
Chicken Farmers of Canada
Canadian Poultry and Egg Processors Council
Executive summary
Food animals including avian species naturally carry pathogens in their intestinal tract that may be transferred on to raw meat products during slaughter and processing. The main objective of this microbiological baseline study was to provide national and current baseline estimates on the prevalence and concentration of Campylobacter and Salmonella in broiler chicken and chicken meat produced in Canada. This information will be used to assess risk management programs including the potential setting of pathogen reduction targets or performance standards.
The design of this study follows a unique farm-to-retail approach to provide baseline data at the farm, processing and retail level. This approach is intended to allow governments and industry to evaluate the effects of current and future interventions at all stages along the food chain.
The detection and enumeration of Salmonella, Campylobacter, and generic E. coli in various sample matrices was performed by an accredited third-party laboratory using United States Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) methods. All bacterial isolates recovered from positive samples are being characterized by PHAC and CFIA laboratories using reference phenotyping and genotyping methods.
The prevalence estimates presented in this report are not fully weighted and thus not considered as the final national prevalence estimates. The statistical process of weighting or applying the appropriate weight to each primary sampling unit will be performed and reported at a later stage to derive more precise estimates. The reported prevalence of Campylobacter on fresh abattoir and retail chicken products was estimated by combining the results of both qualitative and quantitative tests.
The farm component was assessed through the collection and testing of caecal contents of chicken carcasses at slaughter which reflects the contamination status of the flock. A pooled caeca sample was collected from a set of 20 individual birds of the same lot or truck load at slaughter to estimate the prevalence of these foodborne pathogens in flocks and farms. The results of this study show that the prevalence of Campylobacter and Salmonella in broiler chicken lots raised on Canadian farms vary widely over seasons and provinces.
The national prevalence of Salmonella in broiler chicken lots was 25.6% (CI: 24.3% – 26.9%). The lots raised in eastern provinces were colonized more frequently with Salmonella with Ontario demonstrated the highest prevalence with 34.3% (CI: 31.4% – 37.2%). The national prevalence of Campylobacter in broiler chicken lots was 24.1% (CI: 22.8% – 25.4%), but the geographical distribution of positive lots increased gradually towards the west with British Columbia showing the highest prevalence at 41.3% (CI: 37.7% – 44.9%).
The prevalence of Salmonella on whole carcasses and parts processed in federally-registered establishments were significantly different at 16.9% (CI: 15.1% – 18.7%) and 29.6% (CI: 27.4% – 31.7%), respectively. Similarly, the prevalence of Campylobacter was significantly lower on whole carcasses at 27.4% (CI: 25.2% – 29.6%), compared to parts at 39.0% (CI: 36.7% – 41.4%). When analyzed separately, the prevalence of both pathogens in skinless and boneless (SLBL) breasts was not significantly different from the prevalence observed on skin-on and bone-in (SOBI) thighs.
Similar types of raw chicken products were collected from supermarket chains and independent grocers or butcher shops in 33 large cities across Canada. Although a large proportion of retail chickens sold in supermarket chains are supplied by federally-registered establishments, approximately 20% of sampled chickens were purchased from independent grocers or butcher shops who may sell chicken products processed in provincially-inspected plants. The prevalence of Salmonella on whole carcasses and parts was significantly different with 21.0% (CI: 17.1% – 25.0%) and 31.6% (CI: 29.0% – 34.2%), respectively. However, the prevalence of Campylobacter on whole carcasses was 37.9% (CI: 33.1% – 42.6%), but not significantly different from parts with 43.1% (CI: 40.3% – 45.8%). When analyzed separately, the prevalence of both pathogens on SLBL breasts was not significantly different from SOBI thighs.
An important component of the MBS was to estimate bacterial counts on contaminated specimens. Enumerations were performed on all types of chicken meat products collected in abattoirs and food retail outlets. The geometric mean concentration of Salmonella was 0.11 and 0.09 MPN per ml of rinse fluid on abattoir and retail products, respectively. For Campylobacter, the geometric mean concentration was 3.81 and 1.83 CFU/mL on abattoir and retail products, respectively. It is worth noting that Salmonella counts were similar between different types of abattoir or retail products, in contrast to Campylobacter counts that were significantly lower on SLBL breasts whether they were collected in the abattoirs or food retail outlets.
Generic E. coli is used as a measure of fecal contamination in abattoirs. There were respectively 83.4% (CI: 81.1% – 85.7%), 83.9% (CI: 82.2% – 85.7%), and 95.0% (CI: 93.2% – 96.8%) of SLBL breasts, whole carcasses and SOBI thighs contaminated by generic E. coli. The geographic mean concentration was 51.4 CFU of generic E. coli per ml of rinse on abattoir products, reaching 96.1 CFU/mL for SOBI thighs.
In comparison with the 1997-98 poultry MBS, the prevalence of Salmonella on whole carcasses processed in federally-registered establishments significantly decreased from 21.1% (CI: 18.2% – 24.0%) to 16.9% (CI: 15.1% – 18.7%). No testing for Campylobacter was performed during the previous Canadian MBS preventing comparison with the current study.
This national MBS in broiler chicken provides current baseline estimates on the prevalence and concentration of Campylobacter and Salmonella at various stages along the broiler chicken meat supply chain. This information will be used as a science-based foundation by governments, industry and other stakeholders to inform the development of a risk management strategy for the control of Campylobacter and Salmonella in chicken produced in Canada. To achieve further reduction at processing or retail, a future strategy should consider the implementation of new interventions or mitigation measures along the supply chain from primary production to retail levels.
Abbreviations
BPW: buffered peptone water
CFU: colony forming unit
CI: confidence interval
CFIA: Canadian Food Inspection Agency
CMA: census metropolitan area
FPT: federal, provincial and territorial
FSIS: Food Safety and Inspection Service
MBS: microbiological baseline study
MPN: most probable number
P: prevalence
PRI: Pathogen Reduction Initiative
SLBL: skinless and boneless
SOBI: skin-on and bone-in
Introduction
The federal, provincial and territorial (FPT) governments in Canada began developing a Pathogen Reduction Initiative (PRI) for meat and poultry under ministerial direction in July 2009. This was initiated to strengthen the Canadian food safety system with the primary goal of decreasing the incidence and economic impact of foodborne illness by reducing pathogen contamination of meat and poultry. A baseline sampling plan for Canada was introduced at the national stakeholder information session of February 2011 which identified Campylobacter and Salmonella on raw broiler chicken as a priority meat-hazard combination for baseline work. Campylobacter and Salmonella have been known to cause a significant health burden and cost in Canada (Majowicz et al., 2006; Thomas et al., 2006) and serve as continual sources of acute gastroenteritis worldwide (Flint et al., 2005). The lack of representative and harmonized baseline data on these pathogens along the food chain in Canada has been recognized as an important knowledge gap for the development of pathogen reduction strategies.
The USDA Food Safety and Inspection Service (FSIS) and other international food safety regulators have conducted national microbiological baseline studies (MBS) to set and measure progress against pathogen reduction targets or performance standards and to inform the development of risk management programs. FSIS has implemented tighter performance standards for Salmonella and new standards for Campylobacter in poultry beginning July 2011 based on the results of the 2007-08 baseline study in broiler chicken processed in federal plants. Similarly, the UK Food Standards Agency (FSA) set a new target for the reduction of Campylobacter levels on raw chicken in December 2010 following the 2007-08 retail chicken study (FSA, 2009). More recently, FSIS is planning to release new performance standards for both pathogens in chicken parts by the end of the 2015 fiscal year (FSIS, 2013; Food Safety News, 2014). Several strategies and control guidelines have been published by national authorities (FSIS, 2010; EFSA, 2011) and international organizations (FAO/WHO, 2009; CAC, 2011) to reduce Campylobacter and Salmonella along the broiler chain, with proposals to achieve performance targets.
Under the work conducted by the FPT Pathogen Reduction Working Group, the CFIA undertook a national microbiological baseline study to estimate the prevalence and concentration of Campylobacter and Salmonella in broiler chicken and chicken meat produced across Canada. The main objective of this MBS was to estimate the prevalence and concentration of Salmonella and Campylobacter in broiler chicken from farm production to the retail market. This report provides a summary of the study design and associated methodologies as well as a descriptive analysis of microbiological data from the examination of broiler chicken lots, whole carcasses and parts processed in federally-registered establishments and sold in retail food outlets in Canada. The prevalence presented in this report should not be considered as the national prevalence, but rather the proportion of samples that tested positive. The calculated weighted national prevalence estimates for these pathogens will be reported in a separate publication describing the epidemiological and inferential statistical analysis in detail.
Objectives
The main objective of this baseline study was to provide national and current baseline estimates on the prevalence and concentration of Campylobacter and Salmonella in broiler chicken caeca and chicken meat. This information is intended to be used to develop pathogen reduction programs and to serve as benchmarks against which the industry could measure the effectiveness of their HACCP programs and/or intervention measures over time. Such information may also be used to support future risk assessment studies.
Specifically, the study was designed to:
- Establish baseline prevalence and concentration of Salmonella and Campylobacter in caeca of broiler chicken flocks raised on farms across Canada.
- Establish baseline prevalence and concentration of generic E. coli, Salmonella and Campylobacter on broiler chicken carcasses and carcass parts processed in federally-registered establishments.
- Provide estimates on the concentration of Salmonella and Campylobacter in weep fluids of bulk packs containing whole chicken carcasses prior to distribution to hotels, restaurants, and institutions (HRI).
- Establish baseline prevalence and concentration of Salmonella and Campylobacter on raw chicken meat products available on the Canadian retail marketplace.
- Compare new baseline prevalence and concentration data of Salmonella and generic E. coli on broiler chicken carcasses with previous baseline data collected during the 1997-98 study.
- Evaluate the geographical and seasonal distribution of Salmonella and Campylobacter in broiler chicken flocks grown on farms across Canada.
- Compare Salmonella and Campylobacter phenotypes and genotypes encountered at the farm, abattoir, and retail level to compare them to those causing illness in humans.
Study design and sampling methods
A farm-to-retail design was developed to provide baseline prevalence estimates at the farm, processing and retail levels. No farm visits were conducted or environmental samples collected from broiler chicken barns. The farm component was assessed through the collection and testing of caecal contents of chicken carcasses at slaughter to reflect the contamination status of the flock. The selection of federally-registered poultry slaughter establishments was based on the weight class and slaughter volume of chickens from active establishments between January and December 2011. In this study, establishments processing broiler chickens having a live weight greater than 1.4 kg and less than 2.7kgkg with an annual slaughter volume greater than 100,000 birds were included in the sampling frame. This study population constitutes 92.9% of total chicken production and excludes all small Rock Cornish game hens and roasters. Any establishments producing less than 100,000 birds annually of the selected weight class were excluded from the sampling frame. From a total of 45 federally-registered chicken slaughtering establishments listed in 2011, 37 met these criteria, accounted for 93.9% of total chicken slaughter, and were distributed across nine provinces.
The sample size was determined to estimate the prevalence of Campylobacter and Salmonella in the target population and products with a precision of ± 5% of the true value with 95% confidence and based on the probability that 50% of broiler chicken and chicken products would be contaminated. For farm prevalence, the sample size was corrected for the finite population of broiler chicken farms present in each province. The sample size of the target population and products was then adjusted to allow comparison of prevalence data by season. A season is defined as a quarter of the year such as winter corresponds to the months of December, January and February and so on. Within each season, a sampling date from Monday to Thursday (excluding all statutory holidays and two weeks during Christmas) was randomly assigned to each sample. For logistical reasons, the number of samples in a given sampling day was adjusted to allow a maximum of three caeca samples, one whole carcass, one chicken part and one weep for a total of six samples per abattoir.
Slaughter
To estimate the prevalence of Campylobacter and Salmonella in the entire population of Canadian flocks and farms, broiler chicken lots slaughtered in federally-registered establishments were sampled using a multistage sampling method. In each establishment, a sample of the primary units or broiler lots listed in a given sampling day or kill day was randomly selected and then a fixed number of secondary units or viscera packs from that lot were selected. As such, a lot or a truck load of broiler chickens of 1.4 to 2.7 kg live weight was first selected over the entire production day (includes night shifts in multiple-shift plants) from which a set of 20 caeca from individual birds was pooled and tested at the laboratory to confirm the microbial status of the lot. The number of lots sampled in each establishment was calculated based on the total number of farms within the province and the establishment's slaughter volume.
Processing
Whole carcasses and carcass parts produced in federally-registered establishments were randomly selected at different times within a production day to evaluate the prevalence of pathogens and indicator organisms on raw chicken products prior to distribution to the retail market. The selected types of raw chicken meat products sampled at the abattoir were similar to those collected at retail. Whole carcasses were selected at post-chill, while skinless and boneless (SLBL) breasts and skin-on and bone-in (SOBI) thighs packaged in traypacks were preferably collected immediately after packing, or if not available, directly from bulk-pack containers or at the last readily accessible point prior to packing. The number of whole carcass and carcass part samples per establishment were allocated proportionally to their slaughter volume.
In addition, weep fluid from a limited number of bulk packs containing multiple carcasses was sampled to primarily estimate the concentration of selected pathogens in these fluids that might be potential sources of cross-contamination in HRIs. The number of allocated samples in each establishment was proportional to their slaughter volume.
Retail
The retail component was designed to reflect the retail market share with respect to store type and types of chicken products purchased by the Canadian population. It was previously estimated that the main supermarket chains and their affiliates possess 78% of the market share of chicken products in Canada (National Farmers Union, 2005). Retail samples were thus collected from supermarket chains and independent grocers at a 4:1 ratio in the urban area of 33 census metropolitan areas (CMA) across Canada. These CMAs or major cities are formed by one or more adjacent municipalities centred on a large urban area. The total urban area of these CMAs allows the coverage of 62% of the Canadian population based on the 2006 census, while restricting the sampling territory to a manageable level. The number of retail samples allocated in each CMA was based on the population size of each urban area. The random selection of a supermarket chain store or an independent grocery store (including butcher shops) was performed by provincial inspectors (one CMA was sampled by a CFIA inspector) from each CMA using existing provincial or regional databases. Three popular types of raw chicken meat products purchased by Canadian consumers were selected based on a food consumption study (Nesbitt et al., 2008) and were collected at the following frequencies: 50% SLBL breasts, 25% SOBI thighs, and 25% whole chicken carcasses. To limit the number of store visits to a manageable level, the maximum number of samples collected within the same store during a given sampling day ranged from one to three samples depending on the sample size of each CMA.
Sample collection
Samples were collected from federally-registered establishments and retail outlets between December 3, 2012 and December 19, 2013. The collection of caeca samples involved the manual detachment of one intact caecum from a pair of caeca from 20 individual viscera packs of the same broiler lot which was pooled into one composite sample. Collection of whole carcasses was performed according to Annex U of Chapter 11 of the CFIA's Meat Hygiene Manual of Procedures. In brief, a whole carcass was randomly selected and collected after chilling, at the end of the drip line or at the last readily accessible point prior to packing/cut-up using sterile gloves. For carcass parts, tray packs containing > 700 g of SLBL breasts or SOBI thighs were collected immediately after packing or parts were directly taken from a bulk-pack container if tray packs were not available. Weep fluid was collected from bulk packs containing multiple whole chicken carcasses after transferring the carcasses into a new bag provided by the establishment which were then re-packed in the original box. The weep or bloody viscous fluid contained in the original bag was collected by cutting a small opening in a sanitized corner of the bag and carefully draining 200 mL of the weep fluid into a sterile 250 mL wide-mouth plastic jar.
Similarly, tray packs containing >700 g of fresh SLBL chicken breasts or SOBI thighs, or a whole chicken, were randomly selected from the display counters in grocery stores. Whole chicken could either be packaged in a tray pack or in a modified atmosphere bag and weigh between 1 to 2 kg. All samples were packed and shipped at the earliest possible time after collection using overnight delivery service. Samples received at the laboratory within 72 hours after collection and with a surface temperature ranging from − 0.4°C to +10.4°C were accepted for analysis. All samples fit for testing were processed the day of arrival or the following day by 12:00 pm if the samples were received after 5:00 pm the previous day.
Laboratory analysis
Sample preparation
Whole carcasses and parts collected at abattoir and retail were aseptically removed from their packages or sample bags at the third-party laboratory and prepared for a rinsing procedure. All whole carcasses were rinsed according to the FSIS procedure for whole bird rinse whereby each carcass is rinsed inside and out with 400 mL of buffered peptone water (BPW) using a rocking motion for 1 min (CFIA, 2010). For chicken part samples, BPW was added to achieve a final meat to BPW ratio of 4.5 g per mL, and samples were manually massaged by kneading the stomacher bag for 2 min (FSIS, 2010a). The contents of each individual caecum from a sample of 20 caeca was aseptically collected to prevent possible cross-contamination from the external surface of caeca, and pooled to form one composite sample. The composite of caecal content was suspended in BPW in a 1:4 ratio (w/w) and stomached for 2 min. All prepared samples were refrigerated pending testing.
Analytical methods
All samples were screened for Salmonella by the BAX® PCR system using the MLG 4C.03 method (FSIS, 2011a). Depending on the sample type, presumptive positives were either confirmed by the culture method MLG 4.05 (FSIS, 2011b) or enumerated using a combination of MLG 4.05 and a 3-tube Most Probable Number (MPN) procedure as described in MLG Appendix 2.03 (FSIS, 2008). All MPN tubes were cultured and confirmed for Salmonella species according to MLG 4.05. For caeca samples, the concentration of Salmonella was determined in a limited number of positive samples set at a maximum of 36 samples per month. As the concentration of Salmonella in caeca samples was greater than the maximum MPN value in a large proportion of positive caeca samples, the number of dilutions was increased from three to five during the course of the study.
All samples were also tested by the direct agar plating method MLG 41.01 (FSIS, 2010b) for detection and enumeration of Campylobacter with the following modifications. For caeca samples, volumes of 0.1 mL of 10-4 to 10-6 dilutions of caecal contents were plated onto duplicate Campy-Cefex plates. The low dilutions of caecal contents (10-1 and 10-3) were not plated due to the observation of significant overgrowth on these plates during the pilot phase of the study resulting in plates that were not countable. For weep fluids, volumes of 0.1 mL of undiluted weep sample and of the 10-1 to 10-4 dilutions were plated onto duplicate Campy-Cefex plates. Regardless of sample type, five suspect colonies, proportional to all typical colony types observed from one or more plates were picked and confirmed for Campylobacter species. Each colony was examined for characteristic morphology and motility under phase-contrast microscopy. All presumptive positive isolates were pooled and confirmed by latex agglutination assay specific for C. jejuni, C. coli, and C. lari. Enumeration of Campylobacter was conducted for each confirmed pool whereby three colonies for each colony type were individually speciated using a multiplex PCR method specific for C. jejuni and C. coli (Health Canada, 2011). All colonies belonging to confirmed colony types were counted on the appropriate dilution plates. Thus, the Campylobacter counts reported herein are the total count of C. jejuni and C. coli. In addition to direct plating, all carcasses and parts were tested by a qualitative or broth enrichment culture method as described in MLG 41.01.
Detection and enumeration of generic E. coli was only performed on rinse fluids from whole carcasses and parts collected in abattoir according to the MFHPB-34 method (Health Canada, 2001), but using 1 mL of undiluted sample and four serial dilutions (10-1 to 10-4) prepared with BPW (Curiale, 1991).
Statistical analysis
Although the number of allocated samples to each abattoir and CMA was based on production volume and population density, respectively, the prevalence estimates presented in this report are not yet considered fully weighted and thus called "unweighted". The unweighted prevalence is the proportion of positive samples to a pathogen or indicator organism in the sampled population and products. The prevalence estimates are all representative on a national scale, regardless of sample type, with the exception of farm and flock prevalence that are also generated on a provincial/regional basis. To maintain confidentiality of data sources, the province of Manitoba and Saskatchewan were grouped under the Midwest region while Newfoundland, New Brunswick, Nova Scotia and Prince-Edward-Island were grouped under the Maritimes. All statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA) and the significance level was set at p < 0.05. Comparison of prevalence values among product types within a stage of the supply chain and between seasons was done by performing a Chi-Square test. As the log-transformed concentration data for both pathogens was not normally distributed using the Shapiro-Wilks W statistic (p < 0.0001), the Kruskal-Wallis one-way analysis of variance was conducted to determine whether the median concentrations values differ among multiple product types followed with the Wilcoxon-Mann-Witney test for the comparison between individual groups.
Results
Sampling
Of a total of 10,023 planned samples, 9,615 (or 95.9%) were collected and tested, including 7,961 abattoir and 1,654 retail samples indicating a high compliance with the sampling plan from participating establishments and provinces. Table 1 summarizes the number of planned, received and tested samples during the study. More than 91% of planned samples of each sample type were collected and tested with the exception of weep fluid samples with a proportion of 79.4%. Eight of 38 (or 21.1%) federally-registered poultry establishments did not produce bulk packs of whole carcasses during the study which resulted in a lower sampling rate for this sample type. The sampling plan of a single abattoir in Quebec was modified during the course of the study to reflect the reduction in slaughter activities in favour of a new poultry registered establishment from the Maritimes which started production operations in November 2012 and began sampling for the MBS in May 2013. The inclusion of the establishment in the sampling frame increased the total number of establishments to 38.
Many samples were declared unfit for testing at reception mostly due to their long transit time (3.0%) and/or surface temperature exceeding the acceptable temperature range (2.4%). Replacement samples were collected to ensure that at least 90% of planned samples were obtained and tested for each season for valid statistical analysis. Several part samples that were close to or less than 500 g sample weight did not yield sufficient rinse fluid to perform all microbiological tests during the first month of the study, in particular the qualitative test for Campylobacter. It appeared that part of the BPW added to these samples during the rinsing procedure at the laboratory was absorbed by the skin or muscle tissues, particularly by the SOBI thighs. Therefore, the minimum sample weight for part samples was increased to 700 g for the remainder of the study in order to yield sufficient rinse volume for all tests. In addition, we extended the study for one month to December 2013 to compensate for the reduced number of Campylobacter tests done during the winter season. Sample testing results for the month of December 2013 were combined with those for the winter period (December 2012 to February 2013) for analysis.
Slaughter
A total of 4,541 broiler chicken lots originating from various farms across Canada were sampled at slaughter in 38 federally-registered establishments and tested for Campylobacter and Salmonella. The unweighted prevalence or proportion of positive lots for Salmonella in Canada was 25.6% (CI: 24.3% – 26.9%) and ranged from a minimum of 17.4% (CI: 14.4% – 20.5%) in Midwest provinces to a maximum of 34.3% (CI: 31.4% – 37.2%) in Ontario (Table 2); the prevalence being significantly higher in Ontario than any other province or region except in Atlantic region. The geographical distribution of positive lots shows that the provinces from eastern Canada have higher prevalence than those from western Canada. No seasonal pattern was apparent for Salmonella in broiler chicken lots as little variation was observed during the year (Figure 1). The concentration of Salmonella in broiler chicken caeca was found to be highly variable from <0.3 to >11,000 MPN/g of caecal content (Tables 3a and 3b). Of 502 positive caeca samples tested, 64.9% had a concentration greater than 110 MPN/g.
The proportion of positive broiler chicken lots for Campylobacter in Canada was 24.1% (CI: 22.8% – 25.4%) and ranged from a minimum of 15.7% (CI: 13.4% – 18.0%) in Quebec to a maximum of 41.3% (CI: 37.7% – 44.9%) in British Columbia (Table 4); the prevalence being significantly higher in British Columbia than any other province or region. With the exception of the Maritimes, the distribution of positive lots for Campylobacter shows a spatial trend increasing gradually from Quebec towards the western provinces. The analysis of seasonal variation showed a small decrease in proportion of positive lots from winter to spring followed with a significant increase during summer and fall (Figure 2). Of 4,445 caeca samples tested, 3,370 (or 75.8%) samples were found negative or below the limit of detection of 50,000 CFU per g of caecal content. Out of 1,075 quantifiable caeca samples, at least 1,016 (or 96.9%) had a Campylobacter concentration between 106 to 109 CFU per g of caecal content (Table 5).
Processing
A total of 1,643 whole carcasses and 1,668 part samples from abattoirs were tested for the presence of Salmonella (Table 6). The prevalence of Salmonella on whole carcasses was 16.9% (CI: 15.1% - 18.7%) and significantly lower than the 29.6% (CI: 27.4% – 31.7%) prevalence observed in parts. When analyzed separately, the prevalence of Salmonella on SLBL breasts was 28.3% (CI: 25.6% – 31.0%) and not significantly different from the prevalence observed on SOBI thighs with 31.7% (CI: 28.0% – 35.4%).The seasonal variation of Salmonella on fresh abattoir chicken was examined for whole carcasses and parts separately as they have sufficient sample size for valid statistical comparison. There were no clear seasonal patterns for Salmonella, regardless of sample type. The variation of Salmonella prevalence on whole carcasses or parts was relatively small over the study period such that no significant difference in prevalence was observed among all seasons (Figure 1).
Of the 3,333 fresh abattoir chicken products tested, 781 were enumerated and only 11 (or 1.4%) exceeded 11 MPN/mL of rinse fluid (Table 7). The concentration of Salmonella on fresh abattoir chicken was below 3 MPN/mL for 94.9% of samples, of which 164 (or 21.0%) were below the limit of detection. The geometric mean concentration of Salmonella in rinse fluid of fresh abattoir chicken products ranged from 0.10 to 0.12 MPN/mL and there was no statistically significant difference between carcasses and parts or within part types (Tables 8a and 8b).
A total of 1,646 whole carcasses and 1,675 part samples from abattoirs were tested for the presence of Campylobacter using a quantitative and a qualitative method run in parallel. The prevalence of Campylobacter on fresh abattoir chicken was higher by the qualitative method, regardless of the sample type due to the enrichment broth allowing for cell resuscitation (Table 9). By combining results of both tests, the prevalence on whole carcasses was 27.4% (CI: 25.2% – 29.6%) and significantly lower than on parts with 39.0% (CI: 36.7% – 41.4%). The prevalence of Campylobacter on SLBL breasts was 39.0% (CI: 36.0% – 41.9%) and not significantly different from the prevalence observed on SOBI thighs with 39.2% (CI: 35.3% – 43.0%).The analysis of seasonal variation showed a decrease in proportion of positive whole carcass (significant) and part samples from winter to spring followed with a marked and significant increase during summer and fall for both sample types (Figure 2). For instance, the prevalence of Campylobacter on whole carcasses was 19.8% (CI: 15.9% - 23.7%) in spring and increased to 31.2% (CI: 26.7% - 35.7%) in summer.
Overall, 79.0% of fresh abattoir chicken samples were negative for Campylobacter (below the limit of detection, i.e., <1 CFU/mL) by the quantitative method of MLG 41.01 (Table 10). Out of 701 quantifiable samples, at least 346 (or 49.4%) had a concentration below 10 CFU per mL of rinse fluid. The geometric mean concentration of Campylobacter in rinse fluid of fresh abattoir chicken products ranged from 1.98 to 5.65 CFU/mL and was significantly higher on whole carcasses than on parts, but not when compared with SOBI thighs only (Tables 11a and 11b). The concentration of Campylobacter was also significantly higher on SOBI thighs than on SLBL breasts.
Of the 77 weep fluid samples collected from bulk packs containing multiple carcasses, 28 (or 36.4%) were positive for Salmonella and 15 (or 19.5%) for Campylobacter. The concentration of Salmonella in weep fluid was low ranging from below the limit of detection to 2.4 MPN/mL while the concentration of Campylobacter largely varied from undetected to a maximum of 660 CFU/mL.
A total of 1,643 whole carcasses and 1,591 part samples from abattoir were tested for the presence of generic E. coli (Tables 12a, 12b and 12c). The prevalence of generic E. coli on whole carcasses was 83.9% (CI: 82.2% – 85.7%) and 87.6% (CI: 86.0% - 89.2%) on parts. When parts were analyzed separately, the prevalence of generic E. coli on SLBL breasts was 83.4% (CI: 81.1% – 85.7%) and significantly lower than on SOBI thighs with 95.0% (CI: 92.9% – 96.5%). Out of 2,773 quantifiable samples, 2,294 (or 82.7%) had a concentration within the range of 11 to 1000 CFU per mL of rinse fluid. The geometric mean concentration of generic E. coli in rinse fluid of SOBI thighs was 96.1 CFU/mL and significantly higher than those observed on whole carcasses and SLBL breasts with 50.6 CFU/mL and 34.3 CFU/mL, respectively (Tables 13a and 13b).
Retail
A total of 404 whole carcasses and 1,239 part samples from the retail market were tested for the presence of Salmonella (Table 14). The prevalence of Salmonella on whole carcasses was 21.0% (CI: 17.1% – 25.0%), significantly lower than on parts with 31.6% (CI: 29.0% - 34.2%). When analyzed separately, the prevalence of Salmonella on SLBL breasts (P: 31.4%, CI: 28.3% – 34.6%) was not significantly different from that of SOBI thighs (32.1%, CI: 27.6% – 36.6%).The seasonal variation of Salmonella on fresh retail chicken was examined for all products grouped together as the sample size was not sufficient to analyze each sample type individually for valid statistical comparison. No seasonal pattern was apparent for Salmonella on fresh retail chicken products. The prevalence was higher during the spring (P: 32.4%, CI: 27.7% – 37.1%) and lower in summer (P: 25.3%, CI: 21.1% – 29.5%), but no significant difference was observed among all seasons (Figure 1).
The concentration of Salmonella on fresh retail chicken was low on most samples regardless of sample type, with 95.2% below 3 MPN/mL of rinse fluid (Table 15). The geometric mean concentrations of Salmonella in rinse fluid of fresh retail chicken products ranged from 0.07 to 0.09 MPN/mL and no significant difference was observed between carcasses and parts or within part types (Tables 8a and 8b).
A total of 404 whole carcasses and 1,247 part samples from the retail market were tested for the presence of Campylobacter using a quantitative and a qualitative method run in parallel. The prevalence of Campylobacter on fresh retail chicken was higher by the qualitative method, regardless of the sample type (Table 16). By combining results from both tests, the prevalence was 37.9% (CI: 33.1% – 42.6%) on whole carcasses, not significantly lower than on parts with 43.1% (CI: 40.3% – 45.8%). The prevalence of Campylobacter on SLBL breasts (P: 43.3%, CI: 39.9% – 46.6%) was found to be similar to that of SOBI thighs (P: 42.6%, CI: 37.8% – 47.4%).The analysis of seasonal variation shows a small decrease in proportion of positive retail products from winter to spring (P: 30.1%, CI: 25.5% - 34.7%) followed with a significant increase during summer (P: 50.3%, CI: 45.4% - 55.2%) and fall (Figure 2).
Overall, 78.9% of fresh retail chicken samples were negative for Campylobacter (below the limit of detection (<1 CFU/mL) by the quantitative method (Table 17). Out of 348 quantifiable samples, 231 or 66.4% had a concentration below 10 CFU per mL of rinse fluid. The geometric mean concentration of Campylobacter in rinse fluid of fresh retail chicken products ranged from 1.13 to 3.23 CFU/mL and was significantly higher on whole carcasses than on parts (Table 11), but not when compared with SOBI thighs only (Table 11). The concentration of Campylobacter was also significantly higher on SOBI thighs than on SLBL breasts.
Discussion
Overall, 96% of 10,023 planned samples were collected along the broiler supply chain and tested for the presence and enumeration of two foodborne pathogens, Campylobacter and Salmonella, and one indicator organism of meat hygiene, generic E. coli. This high performance in sample collection and testing achieved during the entire study will allow inference of prevalence estimates to the target populations and an evaluation of seasonal variation of microorganisms in broiler chicken flocks, carcasses and parts at the chosen precision level in the study design.
The presence of Campylobacter and Salmonella on broiler chicken carcasses primarily originates from the intestinal carriage in the live birds. Both pathogens colonize the intestinal tracts of broiler chicken and can reach extremely high numbers in caecal content (Newell and Fearnley, 2003; FSANZ, 2010) The microbiota of the caecal contents of slaughtered broiler chickens is commonly used to evaluate the microbial status of flocks or farms (Arsenault et al., 2007; Guerin et al., 2007).The detection of Campylobacter- or Salmonella-positive broiler chicken lots at slaughter was performed by pooling and testing 20 intact caeca from individual birds of the same lot taken after the evisceration process. Any positive pooled caeca samples would primarily reflect the colonisation of lots of broiler chicken that have been raised in the same barn. Planned analysis of barn and producer identifiers will allow us to estimate and report on the prevalence of these pathogens at the flock and farm level.
The national prevalence of Salmonella in broiler chicken lots processed in federally-registered establishments was 25.6% with provinces from eastern Canada showing the highest prevalence ranging from 28.9% to 34.3%. In contrast, the national prevalence of Campylobacter-colonised broiler chicken lots was 24.1% with the higher prevalence observed in the provinces of western Canada, especially in British Columbia at 41.3%. Differences in climatic conditions (Patrick et al., 2004; Jonsson et al., 2012) and/or farm-level factors (Arsenault et al., 2007; Guerin et al., 2007) influencing the risk of Campylobacter colonization of broilers flocks may explain the variation among provinces, but further research is needed to expand on these findings.
The prevalence of Campylobacter, Salmonella and generic E. coli were determined on whole chicken carcasses and parts processed in federally-registered establishments at the national scale and not by province. Sampling of whole carcasses occurred at post-chill similar to the previous 1997-98 MBS (CFIA, 2000). Parts, either SLBL breasts or SOBI thighs were collected as tray packs or directly from a bulk pack prior to packaging when tray packs were not produced. The prevalence of Campylobacter and Salmonella on chicken parts was found to be 1.4 and 1.8 times higher than those observed on whole carcasses, respectively. Cross-contamination events resulting from further processing and handling of parts may explain the differences in prevalence between these types of poultry products as was hypothesized by FSIS (2010a). In comparison with the 1997-98 MBS, the prevalence of Salmonella on whole carcasses significantly decreased from 21.1% to 16.9% (CFIA, 2000). This comparison should be made with caution as the detection methods for Salmonella were not the same and the difference in prevalence observed between the two studies does not provide an accurate measure of temporal trend. No testing for Campylobacter was performed during the previous Canadian MBS preventing comparison with the current study.
Similar types of raw chicken products were collected and tested from supermarket chains and independent grocers or butcher shops in the larger cities across Canada. Although a large proportion of retail chickens sold in supermarket chains are supplied by federally-registered establishments, approximately 20% of sampled chickens were purchased from independent grocers or butcher shops who may offer chicken products processed in provincially-inspected plants. The food retail outlets offer a variety of chicken meat products to consumers that could be processed in federal, provincial, or more rarely in a poultry establishment outside of Canada. These products could be pre-packaged in establishments or packaged in-store, of organic or conventional production, and/or further processed. In this study, all selected products were fresh and excluded products that were cooked, frozen, previously frozen, marinated or seasoned. The prevalence of Campylobacter and Salmonella on fresh retail chicken products was 41.8% (CI: 39.4% – 44.2%) and 29.0% (CI: 26.8% - 31.2%), respectively.
The prevalence of Campylobacter and Salmonella on fresh chicken parts was higher than in whole carcasses whether they were collected in abattoirs or retail food outlets. The concentration of Salmonella was generally low and not different within and between product types. In contrast, the concentration of Campylobacter on SLBL breasts was significantly lower than those found on whole carcasses and SOBI thighs. A large-scale retail study in the UK also showed that the concentration of Campylobacter was higher on parts with the skin-on compared to those with the skin-off (FSA, 2009).
This national MBS in broiler chicken provides current baseline estimates on the prevalence and concentration of Campylobacter and Salmonella at various stages along the broiler chicken meat supply chain. It is recognized that this is a snapshot in time and support for further/continued research may have to be pursued. This information is intended to be used as a science-based foundation by governments, industry and other stakeholders to inform the development of a risk management strategy for the control of Campylobacter and Salmonella in chicken produced in Canada. To achieve further reduction at processing or retail, a future strategy should consider the implementation of new interventions or mitigation measures along the supply chain from primary production to retail levels.
Conclusion and recommendations
The performance in sample collection and testing achieved during the study will allow the estimation of national weighted prevalence of Campylobacter and Salmonella in broiler chicken and chicken meat produced and sold in Canada. Multiple regression analyses will be performed to identify statistical associations among the presence of Campylobacter or Salmonella on samples and potential predictors such as generic E. coli, season, region, establishment size, age and live weight of birds, production shift and chilling systems. The diversity of bacterial isolates recovered along the supply chain for broiler chicken will be determined using reference phenotyping and genotyping methods and compared to that of human isolates. Based on the key findings of the study, some recommendations and next steps have been formulated to initiate the discussion on elements that could be considered for the elaboration of a risk management strategy.
- Engage governments, industry and other stakeholders on the evaluation of the microbiological status of Canadian chicken production along the food continuum and collectively determine the path forward for the development and implementation of a risk management strategy.
- Support research initiatives to study the risk factors that influence the colonization with Campylobacter and Salmonella of broiler chicken lots raised in different regions.
- Identify best practices and science-based cost-effective interventions for the control of Campylobacter and Salmonella along the supply chain for broiler chicken from primary production through retail.
- Support research initiatives to evaluate the applicability of on-farm and processing interventions on Canadian poultry farms and slaughter establishments.
- Support qualitative risk assessment work and the development of a model for evaluating intervention strategies for the reduction of Campylobacter and Salmonella in the supply chain for broiler chicken and setting voluntary reduction targets.
Tables
Table 1 Number of planned, received and tested samples during the study
| Sample type Table Note 1 | Location | Planned samples | Received samples | Tested samples | Performance Table Note 2 |
|---|---|---|---|---|---|
| Caeca | Abattoir | 4,732 | 4,722 | 4,541 | 96.0% |
| Whole carcass | Abattoir | 1,688 | 1,707 | 1,646 | 97.5% |
| SLBL breasts | Abattoir | 1,163 | 1,104 | 1,062 | 91.3% |
| SOBI thighs | Abattoir | 597 | 630 | 613 | 102.7% |
| Weep | Abattoir | 97 | 80 | 77 | 79.4% |
| Whole carcass | Retail | 427 | 412 | 404 | 94.6% |
| SLBL breasts | Retail | 881 | 862 | 841 | 95.5% |
| SOBI thighs | Retail | 438 | 419 | 406 | 92.7% |
| Total Table Note 3 | 10,023 | 9,963 | 9,615 | 95.9% |
Table Notes
- Table Note 1
-
SLBL: skinless and boneless; SOBI: skin-on and bone-in.
- Table Note 2
-
Performance means the proportion of tested samples relative to the number of planned samples.
- Table Note 3
-
Includes 25 abattoir and three retail chicken parts that were not SLBL breasts or SOBI thighs.
Table 2 Unweighted prevalence of Salmonella in broiler chicken lots by province/region
| Province | Lots | Positives | P (%) | 95% CI |
|---|---|---|---|---|
| British Columbia | 743 | 143 | 19.2 | 16.4 – 22.1 |
| Alberta | 584 | 104 | 17.8 | 14.7 – 20.9 |
| Midwest | 596 | 104 | 17.4 | 14.4 – 20.5 |
| Ontario | 1,032 | 354 | 34.3 | 31.4 – 37.2 |
| Quebec | 997 | 288 | 28.9 | 26.1 – 31.7 |
| Maritimes | 395 | 118 | 29.9 | 25.4 – 34.4 |
| Canada | 4,347 | 1,111 | 25.6 | 24.3 – 26.9 |
Table 3a Distribution of Salmonella concentrations (MPN/g) Table Note 4 in broiler chicken caeca (Combined results from three and five dilutions Table Note 5)
| Range | No. of samples | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <0.3 | 10 | 2.0% | 10 | 2.0% |
| 0.3-0.99 | 32 | 6.4% | 42 | 8.4% |
| 1-10 | 70 | 13.9% | 112 | 22.3% |
| 11-110 | 64 | 12.7% | 176 | 35.1% |
| >110 | 326 | 64.9% | 502 | 100.0% |
Table Notes
- Table Note 4
-
Most probable number per g of caecal content.
- Table Note 5
-
Because the proportion of samples >110 MPN/g significantly increased during the course of the study, the number of dilutions was increased to five during the month of June 2013 until the end of the study.
Table 3b Distribution of Salmonella concentrations (MPN/g) Table Note 6 in broiler chicken caeca (Results from five dilutions >110 MPN/g Table Note 7)
| Range | No. of samples | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| 111-1,000 | 63 | 32.1% | 63 | 32.1% |
| 1,001-11,000 | 85 | 43.4% | 148 | 75.5% |
| >11,000 | 48 | 24.5% | 196 | 100.0% |
Table Notes
- Table Note 6
-
Most probable number per g of caecal content.
- Table Note 7
-
Because the proportion of samples >110 MPN/g significantly increased during the course of the study, the number of dilutions was increased to five during the month of June 2013 until the end of the study.
Table 4 Unweighted prevalence of Campylobacter in broiler chicken lots by province/region
| Province | Lots | Positives | P (%) | 95% CI |
|---|---|---|---|---|
| British Columbia | 726 | 300 | 41.3 | 37.7 – 44.9 |
| Alberta | 573 | 145 | 25.3 | 21.7 – 28.9 |
| Midwest | 579 | 131 | 22.6 | 19.2 – 26.0 |
| Ontario | 1,012 | 203 | 20.1 | 17.6 – 22.5 |
| Quebec | 973 | 153 | 15.7 | 13.4 – 18.0 |
| Maritimes | 390 | 93 | 23.8 | 19.6 – 28.1 |
| Canada | 4,253 | 1,025 | 24.1 | 22.8 – 25.4 |
Table 5 Distribution of Campylobacter concentrations (CFU/g) Table Note 8 in broiler chicken caeca
| Range | No. of samples | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <50,000 | 3,370 | 75.8% | 3,370 | 75.8% |
| 50,000-100,000 | 4 | 0.1% | 3,374 | 75.9% |
| 100,001-1,000,000 | 12 | 0.3% | 3,386 | 76.2% |
| 1,000,001-10,000,000 | 93 | 2.1% | 3,479 | 78.3% |
| 10,000,001-100,000,000 | 465 | 10.5% | 3,944 | 88.7% |
| 100,000,001-1,000,000,000 | 458 | 10.3% | 4,402 | 99.0% |
| 1,000,000,001-10,000,000,000 | 16 | 0.4% | 4,418 | 99.4% |
| Undetermined counts Table Note 9 | 27 | 0.6% | 4,445 | 100.0% |
Table Notes
- Table Note 8
-
Colony forming unit per g of caecal content.
- Table Note 9
-
Counts reported as too numerous to count or spreaders reported as greater than values within the 300,000 to 3,000,000,000 CFU/g range.
Table 6 Unweighted prevalence of Salmonella on fresh abattoir chicken
| Sample type Table Note 10 | Samples | Positives | P (%) | 95% CI |
|---|---|---|---|---|
| Whole carcass | 1,643 | 278 | 16.9 | 15.1 – 18.7 |
| SLBL breasts | 1,056 | 299 | 28.3 | 25.6 - 31.0 |
| SOBI thighs | 612 | 194 | 31.7 | 28.0 – 35.4 |
| Parts | 1,668 | 493 | 29.6 | 27.4 – 31.7 |
| All products | 3,333 | 780 | 23.4 | 22.0 – 24.8 |
Table Note
- Table Note 10
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh abattoir samples tested including a few parts (22) that were not SLBL breasts or SOBI thighs.
Table 7 Distribution of Salmonella concentrations (MPN/mL) Table Note 11 on fresh abattoir chicken
| Range | No. of samples Table Note 12 | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <0.03 | 164 | 21.0% | 164 | 21.0% |
| 0.03-0.29 | 414 | 53.0% | 578 | 74.0% |
| 0.3-2.99 | 163 | 20.9% | 741 | 94.9% |
| 3.00-10.99 | 29 | 3.7% | 770 | 98.6% |
| >11 | 11 | 1.4% | 781 | 100.0% |
Table Notes
- Table Note 11
-
Most probable number per mL of rinse fluid.
- Table Note 12
-
Samples include whole carcasses, SLBL breasts, SOBI thighs and a few parts (9) that were not SLBL breasts or SOBI thighs.
Table 8a Enumeration of Salmonella in caeca, fresh abattoir and fresh retail chicken meat products (MPN/mL of rinse fluid)
| Sample type Table Note 13 | Location | Quantifiable samples Table Note 14 | Median | Mean log | Geo mean | Geo mean 95% CI |
|---|---|---|---|---|---|---|
| Caeca | Abattoir | 493 | NA | NA | NA | NA |
| Whole carcass | Abattoir | 276 | 0.092 | -0.92 | 0.12 | 0.10 - 0.15 |
| SLBL breasts | Abattoir | 300 | 0.092 | -0.94 | 0.12 | 0.10 - 0.14 |
| SOBI thighs | Abattoir | 195 | 0.092 | -0.98 | 0.10 | 0.08 - 0.13 |
| Parts | Abattoir | 495 | 0.092 | -0.95 | 0.11 | 0.10 - 0.13 |
| All products | Abattoir | 780 | 0.092 | -0.94 | 0.11 | 0.10 - 0.13 |
| Whole carcass | Retail | 85 | 0.036 | -1.13 | 0.07 | 0.05 - 0.11 |
| SLBL breasts | Retail | 262 | 0.074 | -1.03 | 0.09 | 0.08 - 0.12 |
| SOBI thighs | Retail | 130 | 0.092 | -1.04 | 0.09 | 0.07 - 0.12 |
| Parts | Retail | 392 | 0.074 | -1.03 | 0.09 | 0.08 - 0.11 |
| All products | Retail | 477 | 0.074 | -1.05 | 0.09 | 0.08 - 0.10 |
Table Notes
- Table Note 13
-
Parts mean the total of SLBL breasts and SOBI thighs tested; all products correspond to all fresh abattoir or retail samples tested including a few (9) parts that were not SLBL breasts or SOBI thighs.
- Table Note 14
-
These descriptive statistics also include many positive samples confirmed by culture for which the MPN values were either below the LOD or greater the MPN upper limit; they were respectively replaced by 0.5 times the limit of detection (LOD) and the upper limit value.
Table 8b Enumeration of Salmonella in caeca, fresh abattoir and fresh retail chicken meat products (MPN/g of sample)
| Sample type Table Note 15 | Location | Quantifiable samples Table Note 16 | Median | Mean log | Geo mean | Geo mean 95% CI |
|---|---|---|---|---|---|---|
| Caeca | Abattoir | 493 | 110 | 2.13 | 133.7 | 102.9 – 173.7 |
| Whole carcass | Abattoir | 276 | 0.02 | -1.49 | 0.03 | 0.03 - 0.04 |
| SLBL breasts | Abattoir | 300 | 0.04 | -1.28 | 0.05 | 0.04 - 0.06 |
| SOBI thighs | Abattoir | 195 | 0.04 | -1.34 | 0.05 | 0.04 - 0.06 |
| Parts | Abattoir | 495 | 0.04 | -1.30 | 0.05 | 0.04 - 0.06 |
| All products | Abattoir | 780 | 0.03 | -1.37 | 0.04 | 0.04 - 0.05 |
| Whole carcass | Retail | 85 | 0.01 | -1.69 | 0.02 | 0.01 - 0.03 |
| SLBL breasts | Retail | 262 | 0.03 | -1.35 | 0.04 | 0.04 - 0.06 |
| SOBI thighs | Retail | 130 | 0.03 | -1.42 | 0.04 | 0.03 - 0.05 |
| Parts | Retail | 392 | 0.03 | -1.37 | 0.04 | 0.04 - 0.05 |
| All products | Retail | 477 | 0.02 | -1.43 | 0.04 | 0.03 - 0.04 |
Table Notes
- Table Note 15
-
Parts mean the total of SLBL breasts and SOBI thighs tested; all products correspond to all fresh abattoir or retail samples tested including a few (9) parts that were not SLBL breasts or SOBI thighs.
- Table Note 16
-
These descriptive statistics also include many positive samples confirmed by culture for which the MPN values were either below the LOD or greater the MPN upper limit; they were respectively replaced by 0.5 time the limit of detection (LOD) and the upper limit value.
Table 9 Unweighted prevalence of Campylobacter on fresh abattoir chicken
| Sample type Table Note 17 | Agar plating n | Agar plating P (%) | Enrich. n | Enrich. P (%) | Comb. Tests n | Comb. Tests P (%) | Comb. Tests 95% CI |
|---|---|---|---|---|---|---|---|
| Whole carcass | 1,646 | 19.0 | 1,645 | 23.2 | 1,646 | 27.4 | 25.2 – 29.6 |
| SLBL breasts | 1,062 | 21.3 | 1,008 | 34.2 | 1,062 | 39.0 | 36.0 – 41.9 |
| SOBI thighs | 613 | 25.8 | 568 | 33.1 | 613 | 39.2 | 35.3 – 43.0 |
| Parts | 1,675 | 22.9 | 1,576 | 33.8 | 1,675 | 39.0 | 36.7 – 41.4 |
| All products Table Note 17 | 3,343 | 21.0 | 3,243 | 28.4 | 3,343 | 33.3 | 31.7 – 34.9 |
Table Note
- Table note 17
-
Parts mean the total of SLBL breasts and SOBI thighs tested; all products correspond to all fresh abattoir samples tested including a few (22) parts that were not SLBL breasts or SOBI thighs.
Enrich.: Enrichment, Comb. test: Combined test
Table 10 Distribution of Campylobacter concentrations (CFU/mL) Table Note 18 on fresh abattoir chicken
| Range | No. of samples Table Note 19 | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <1 | 2,642 | 79.0% | 2,642 | 79.0% |
| 1-10 | 346 | 10.3% | 2,988 | 89.4% |
| 10.01-100 | 243 | 7.3% | 3,231 | 96.6% |
| 100.01-1,000 | 77 | 2.3% | 3,308 | 99.0% |
| >1,000 | 9 | 0.3% | 3,317 | 99.2% |
| Undetermined counts Table Note 20 | 26 | 0.8% | 3,343 | 100.0% |
Table Notes
- Table Note 18
-
Colony-forming unit per mL of rinse fluid.
- Table Note 19
-
Samples include whole carcasses, SLBL breasts, SOBI thighs and a few parts (22) that were not SLBL breasts or SOBI thighs.
- Table Note 20
-
Undetermined counts due to spreaders reported as greater than values within 2 to 160 CFU/mL range.
Table 11a Enumeration of Campylobacter in caeca, fresh abattoir and retail chicken meat products (CFU/mL of rinse fluid)
| Sample type Table Note 21 | Location | Quantifiable samples Table Note 22 | Median | Mean log | Geo mean | Geo mean 95% CI |
|---|---|---|---|---|---|---|
| Caeca | Abattoir | 1,075 | NA | NA | NA | NA |
| Whole carcass | Abattoir | 450 | 5.5 | 0.75 | 5.65 | 4.62 - 6.92 |
| SLBL breasts | Abattoir | 414 | 1.0 | 0.30 | 1.98 | 1.70 - 2.31 |
| SOBI thighs | Abattoir | 240 | 6.0 | 0.74 | 5.55 | 4.21 - 7.32 |
| Parts | Abattoir | 654 | 2.0 | 0.46 | 2.89 | 2.50 - 3.34 |
| All products | Abattoir | 1,112 | 3.0 | 0.58 | 3.81 | 3.37 - 4.30 |
| Whole carcass | Retail | 153 | 1.0 | 0.51 | 3.23 | 2.32 - 4.51 |
| SLBL breasts | Retail | 364 | 0.5 | 0.05 | 1.13 | 1.00 - 1.28 |
| SOBI thighs | Retail | 173 | 2.0 | 0.48 | 3.01 | 2.25 - 4.02 |
| Parts | Retail | 537 | 0.5 | 0.19 | 1.55 | 1.36 - 1.77 |
| All products | Retail | 691 | 1.0 | 0.26 | 1.83 | 1.61 - 2.08 |
Table Notes
- Table Note 21
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh abattoir or retail samples tested including a few (9) parts that were not SLBL breasts or SOBI thighs.
- Table Note 22
-
These descriptive statistics also include positive samples confirmed by culture for which counts were either below the LOD or greater than a specific value; they were respectively replaced by 0.5 LOD and such specific value.
Table 11b Enumeration of Campylobacter in caeca, fresh abattoir and retail chicken meat products (CFU / g of sample)
| Sample type Table Note 23 | Location | Quantifiable samples Table Note 24 | Median | Mean log | Geo mean | Geo mean 95% CI |
|---|---|---|---|---|---|---|
| Caeca | Abattoir | 1,075 | 8.60E+7 | 7.86 | 7.28E+7 | 6.62E+7 - 8.02E+7 |
| Whole carcass | Abattoir | 450 | 1.22 | 0.10 | 1.26 | 1.03 - 1.54 |
| SLBL breasts | Abattoir | 414 | 0.22 | -0.36 | 0.44 | 0.38 - 0.51 |
| SOBI thighs | Abattoir | 240 | 1.33 | 0.09 | 1.23 | 0.94 - 1.63 |
| Parts | Abattoir | 654 | 0.44 | -0.19 | 0.64 | 0.55 - 0.74 |
| All products | Abattoir | 1,112 | 0.67 | -0.07 | 0.85 | 0.75 - 0.95 |
| Whole carcass | Retail | 153 | 0.22 | -0.14 | 0.72 | 0.52 - 1.00 |
| SLBL breasts | Retail | 364 | 0.11 | -0.60 | 0.25 | 0.22 - 0.28 |
| SOBI thighs | Retail | 173 | 0.44 | -0.17 | 0.67 | 0.50 - 0.89 |
| Parts | Retail | 537 | 0.11 | -0.46 | 0.34 | 0.30 - 0.39 |
| All products | Retail | 691 | 0.22 | -0.39 | 0.41 | 0.36 - 0.46 |
Table Notes
- Table Note 23
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh abattoir or retail samples tested including a few (9) parts that were not SLBL breasts or SOBI thighs.
- Table Note 24
-
These descriptive statistics also include positive samples confirmed by culture for which counts were either below the LOD or greater than a specific value; they were respectively replaced by 0.5 LOD and such specific value.
Table 12a Distribution of generic E. coli concentrations (CFU/mL) Table Note 25 on fresh abattoir chicken (Whole carcass)
| Range | No. of samples | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <5 | 264 | 16.1% | 264 | 16.1% |
| 5-10 | 219 | 13.3% | 483 | 29.4% |
| 11-100 | 776 | 47.2% | 1,259 | 76.6% |
| 101-1,000 | 353 | 21.5% | 1,612 | 98.1% |
| >1,000 | 31 | 1.9% | 1,643 | 100.0% |
Table Note
- Table Note 25
-
Colony-forming unit per mL of rinse fluid.
Table 12b Distribution of generic E. coli concentrations (CFU/mL) Table Note 26 on fresh abattoir chicken (SLBL breasts)
| Range | No. of samples | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <5 | 168 | 16.6% | 168 | 16.6% |
| 5-10 | 158 | 15.6% | 326 | 32.2% |
| 11-100 | 554 | 54.7% | 880 | 86.9% |
| 101-1,000 | 127 | 12.5% | 1,007 | 99.4% |
| >1,000 | 6 | 0.6% | 1,013 | 100.0% |
Table Note
- Table Note 26
-
Colony-forming units per mL of rinse fluid.
Table 12c Distribution of generic E. coli concentrations (CFU/mL) Table Note 27 on fresh abattoir chicken (SOBI thighs)
| Range | No. of samples | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <5 | 29 | 5.0% | 29 | 5.0% |
| 5-10 | 33 | 5.7% | 62 | 10.7% |
| 11-100 | 257 | 44.5% | 319 | 55.2% |
| 101-1,000 | 227 | 39.3% | 546 | 94.5% |
| >1,000 | 32 | 5.5% | 578 | 100.0% |
Table Note
- Table Note 27
-
Colony-forming unit per mL of rinse fluid.
Table 13a Enumeration of generic E. coli on fresh abattoir chicken meat products (CFU/mL of rinse fluid)
| Sample type Table Note 28 | Quantifiable samples | Median | Mean log | Geo mean | Geo mean 95% CI |
|---|---|---|---|---|---|
| Whole carcass | 1,379 | 45 | 1.70 | 50.6 | 47.2 - 54.4 |
| SLBL breasts | 845 | 30 | 1.54 | 34.3 | 31.8 - 37.1 |
| SOBI thighs | 549 | 90 | 1.98 | 96.1 | 85.2 - 108.3 |
| Parts | 1,394 | 45 | 1.71 | 51.5 | 47.9 - 55.3 |
| All products | 2,795 | 45 | 1.71 | 51.4 | 48.8 - 54.0 |
Table Notes
- Table Note 28
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh abattoir samples tested including a few parts (22) that were not SLBL breasts or SOBI thighs.
Table 13b Enumeration of generic E. coli on fresh abattoir chicken meat products (CFU/g of sample)
| Sample type Table Note 29 | Quantifiable samples | Median | Mean log | Geo mean | Geo mean 95% CI |
|---|---|---|---|---|---|
| Whole carcass | 1,379 | 10.0 | 1.05 | 11.3 | 10.5 - 12.1 |
| SLBL breasts | 845 | 6.7 | 0.88 | 7.6 | 7.1 - 8.3 |
| SOBI thighs | 549 | 20.0 | 1.33 | 21.3 | 18.9 - 24.1 |
| Parts | 1,394 | 10.0 | 1.06 | 11.4 | 10.7 - 12.3 |
| All products | 2,795 | 10.0 | 1.06 | 11.4 | 10.9 - 12.0 |
Table Note
- Table Note 29
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh abattoir samples tested including a few parts (22) that were not SLBL breasts or SOBI thighs.
Table 14 Unweighted prevalence of Salmonella on fresh retail chicken
| Sample type Table Note 30 | Samples | Positives | P (%) | 95% CI |
|---|---|---|---|---|
| Whole carcass | 404 | 85 | 21.0 | 17.1 - 25.0 |
| SLBL breasts | 834 | 262 | 31.4 | 28.3 - 34.6 |
| SOBI thighs | 405 | 130 | 32.1 | 27.6 - 36.6 |
| Parts | 1,239 | 392 | 31.6 | 29.0 - 34.2 |
| All products | 1,646 | 477 | 29.0 | 26.8 - 31.2 |
Table Note
- Table Note 30
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh retail samples tested including a few (3) parts that were not SLBL breasts or SOBI thighs.
Table 15 Distribution of Salmonella concentrations (MPN/mL) Table Note 31 on fresh retail chicken
| Range | No. of samples Table Note 32 | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <0.03 | 133 | 27.9% | 133 | 27.9% |
| 0.03-0.29 | 238 | 49.9% | 371 | 77.8% |
| 0.3-2.99 | 83 | 17.4% | 454 | 95.2% |
| 3.00-10.99 | 15 | 3.1% | 469 | 98.3% |
| >11 | 8 | 1.7% | 477 | 100.0% |
Table Notes
- Table Note 31
-
Most probable number per mL of rinse fluid.
- Table Note 32
-
Samples include whole carcasses, SLBL breasts, SOBI thighs and a few parts (3) that were not SLBL breasts or SOBI thighs.
Table 16 Unweighted prevalence of Campylobacter on fresh retail chicken
| Sample type Table Note 33 | Agar plating n | Agar plating P (%) | Enrich. n | Enrich. P (%) | Comb. tests n | Comb. Tests P (%) | Comb. tests 95% CI |
|---|---|---|---|---|---|---|---|
| Whole carcass | 404 | 22.0 | 402 | 34.3 | 404 | 37.9 | 33.1 - 42.6 |
| SLBL breasts | 841 | 17.6 | 793 | 40.7 | 841 | 43.3 | 39.9 - 46.6 |
| SOBI thighs | 406 | 27.3 | 382 | 34.6 | 406 | 42.6 | 37.8 - 47.4 |
| Parts | 1,247 | 20.8 | 1,175 | 38.7 | 1,247 | 43.1 | 40.3 - 45.8 |
| All products | 1,654 | 21.1 | 1,579 | 37.6 | 1,654 | 41.8 | 39.4 - 44.2 |
Table Note
- Table Note 33
-
Parts mean the total of SLBL breasts and SOBI thighs tested. All products correspond to all fresh abattoir samples tested including a few parts (1) that were not SLBL breasts or SOBI thighs.
Enrich.: Enrichment, Comb. test: Combined test
Table 17 Distribution of Campylobacter concentrations (CFU/mL) Table Note 34 on fresh retail chicken
| Range | No. of samples Table Note 35 | Percent of total | Cumulative number | Cumulative percent |
|---|---|---|---|---|
| <1 | 1,303 | 78.9% | 1,303 | 78.9% |
| 1-10 | 231 | 14.0% | 1,534 | 92.9% |
| 10.01-100 | 84 | 5.1% | 1,618 | 98.0% |
| 100.01-1,000 | 16 | 1.0% | 1,634 | 99.0% |
| >1,000 | 4 | 0.2% | 1,638 | 99.2% |
| Undetermined counts Table Note 36 | 13 | 0.8% | 1,651 | 100.0% |
Table Notes
- Table Note 34
-
Colony-forming units per mL of rinse fluid.
- Table Note 35
-
Samples include whole carcasses, SLBL breasts, SOBI thighs and a few parts (22) that were not SLBL breasts or SOBI thighs.
- Table Note 36
-
Undetermined counts due to spreaders reported as greater than values within 2 to 160 CFU/mL range.
Figures
Figure 1. Prevalence of Salmonella among broiler chicken samples by season; error bars are 95% confidence intervals.

Description for Figure 1
Figure 1. shows the prevalence of Salmonella among broiler chicken samples by season; error bars are 95% confidence intervals.
Winter
- Broiler lots 27.3%, margin of error 2.6%
- Abattoir carcasses 17.5%, margin of error 3.7%
- Abattoir parts 30.1%, margin of error 4.2%
- All retail products 29.5%, margin of error 4.2%
Spring
- Broiler lots 25.5%, margin of error 2.5%
- Abattoir carcasses 16.3%, margin of error 3.6%
- Abattoir parts 26.7%, margin of error 4.3%
- All retail products 32.5%, margin of error 4.7%
Summer
- Broiler lots 24.6%, margin of error 2.5%
- Abattoir carcasses 17.7%, margin of error 3.7%
- Abattoir parts 30.4%, margin of error 4.5%
- All retail products 25.3%, margin of error 4.2%
Fall
- Broiler lots 26.0%, margin of error 2.5%
- Abattoir carcasses 16.2%, margin of error 3.5%
- Abattoir parts 31.0%, margin of error 4.5%
- All retail products 29.0%, margin of error 4.4%
Figure 2. Prevalence of Campylobacter among broiler chicken samples by season; error bars are 95% confidence intervals.
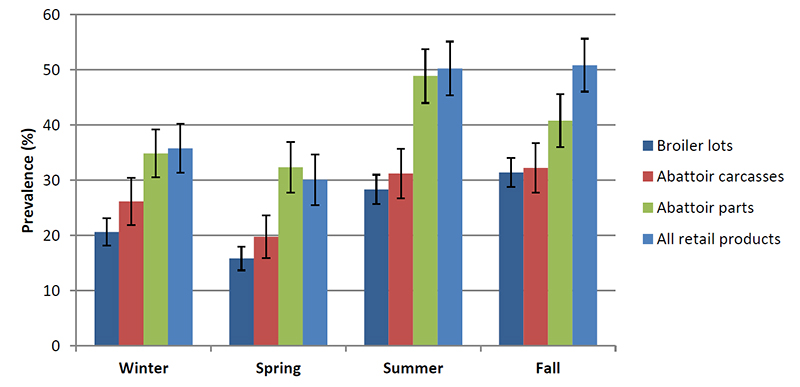
Description for Figure 2
Figure 2 shows the prevalence of Campylobacter among broiler chicken samples by season; error bars are 95% confidence intervals.
Winter
- Broiler lots 20.6%, margin of error 2.5%
- Abattoir carcasses 26.2%, margin of error 4.3%
- Abattoir parts 34.8%, margin of error 4.3%
- All retail products 35.8%, margin of error 4.4%
Spring
- Broiler lots 15.8%, margin of error 2.1%
- Abattoir carcasses 19.8%, margin of error 3.9%
- Abattoir parts 32.3%, margin of error 4.6%
- All retail products 30.1%, margin of error 4.6%
Summer
- Broiler lots 28.4%, margin of error 2.7%
- Abattoir carcasses 31.2%, margin of error 4.5%
- Abattoir parts 48.9%, margin of error 4.9%
- All retail products 50.2%, margin of error 4.9%
Fall
- Broiler lots 31.5%, margin of error 2.7%
- Abattoir carcasses 32.2%, margin of error 4.5%
- Abattoir parts 40.8%, margin of error 4.8%
- All retail products 50.8%, margin of error 4.8%
References
Arsenault, J., Letellier, A., Quessy, S., Normand, V., and Boulianne, M. (2007). Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev. Vet. Med. 81: 250-264.
CAC (2011). Guidelines for the control of Campylobacter and Salmonella in chicken meat.
CODEX Alimentarius
CFIA (2000). Canadian microbiological baseline survey of young chicken and young turkey carcasses - June 1997-May 1998. Canadian Food Inspection Agency, Food of animal origin division, Ottawa.
CFIA (2010). Meat hygiene manual of procedures.
Curiale, M.S., Sons, T., McIver, D., McAllister, J.S., Halsey, B., Roblee, B., and Fox, T.L. (1991). Dry rehydratable film for enumeration of total coliforms and Escherichia coli in foods: collaborative study. J. Assoc. Off. Anal. Chem. 74: 635-648.
EFSA (2010). Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella in broiler carcasses in the EU, 2008. Part A: Campylobacter and Salmonella prevalence estimates. Part A: Campylobacter and Salmonella prevalence estimates. PDF (1.36 MB)
EFSA (2011). Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011; 9(4) 2105.
FAO/WHO (2011). Salmonella and Campylobacter in chicken meat. PDF (433.27 kb)
Flint, J.A., Van Duynhoven, Y.T., Angulo, F.J., DeLong, S.M., Braun, P., Kirk, M., Scallan, E., Fitzgerald, M., Adak, G.K., Sockett, P., Ellis, A., Hall, G., Gargouri, N., Walke, H., and Braam, P. (2005). Estimating the burden of acute gastroenteritis, foodborne disease, and pathogens commonly transmitted by food: an international review. Clin. Infect. Dis. 41: 698-704.
FSIS (2008). Most probable number procedure and tables, MLG Appendix 2.03, Microbiology Laboratory Guidebook, USDA.
FSIS (2010). Compliance guideline for controlling Salmonella and Campylobacter in poultry PDF (1.36 mb)
FSIS (2010a). Nationwide raw chicken parts microbiological baseline data collection program. PDF (407 KB)
FSIS (2010b). Isolation, identification, and enumeration of Campylobacter jejuni/coli/lari from poultry rinse and sponge samples, MLG Appendix 41.00, Microbiology Laboratory Guidebook, USDA.
FSIS (2011a). FSIS procedure for the use of a polymerase chain reaction (PCR) assay for screening Salmonella in raw meat products, raw catfish products, carcass sponge samples, whole bird rinses, ready-to-eat meat, poultry products, and pasteurized egg products, MLG Appendix 4C.03, Microbiology Laboratory Guidebook, USDA.
FSIS (2011b). Isolation and identification of Salmonella from meat, poultry, pasteurized egg and catfish products, MLG Appendix 4.053, Microbiology Laboratory Guidebook, USDA.
FSIS (2013). Salmonella Action Plan – Strategic Performance Working Group.
Food Safety News (2014). FSIS drafting Campylobacter performance standards for chicken parts.
Guerin, M.T., Martin, W., Reiersen, J., Berke, O., McEwen, S.A., Bisaillon, J.-R., and Lowman, R (2007). A farm-level study of risk factors associated with the colonization of broiler flocks with Campylobacter spp. in Iceland, 2001-2004. Act. Vet. Scand. 49 (I8): 1-12.
Health Canada (2001). MFHPB-34 method - Enumeration of E. coli And Coliforms In Food Products And Food Ingredients Using 3M™ Petrifilm™ E. coli Count Plates, MFHPB-34, Compendium of Analytical Methods, Health Canada.
Health Canada (2011). Laboratory procedure (unpublished) - Speciation of presumptive Campylobacter jejuni and C. coli colonies by multiplex Polymerase Chain Reaction (mPCR), Ottawa.
Jonsson, M.E., Chriél, M., Norström, M., and Hofshagen, M. (2012). Effect of climate and farm environment on Campylobacter spp. colonisation in Norwegian broiler flocks. Prev. Vet. Med. 107: 95-104.
Majowicz, S.E., McNab, W.B., Sockett, P., Henson, S., Doré, K., Edge, V.L., Buffett, M.C., Fazil, A., Read, S., McEwen, S., Stacey, D., and Wilson, J.B. (2006). Burden and cost of gastroenteritis in a Canadian community. J. Food Prot. 69: 651-659.
National Farmers Union (2005). Canada food retail – 2004/2005. Supermarket revenues and market shares previously published.
Newell, D.G., and Fearnley, C. (2003). Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69: 4343-4351.
Patrick, M., Christiansen, L., Wainø, M., Ethelberg, S., Madsen, H., and Wegener, H. (2004). Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl. Environ. Microbiol. 70: 7474-7480.
Thomas, M.K, Majowicz, S.E., Sockett, P.N., Fazil, A., Pollari, F., Doré, K., Flint, J.A., and Edge, V.L. (2006). Estimated numbers of community cases of illness due to Salmonella, Campylobacter and verotoxigenic Escherichia coli: pathogen-specific community rates. Can. J. Infect. Dis. Med. Microbiol. 17: 229-234.
- Date modified: