Legibility and location of food labelling information
On this page
Legibility requirements
General
As per section A.01.016 of the Food and Drugs Regulations (FDR) and section 208 of the Safe Food for Canadians Regulations (SFCR), mandatory information required to appear on the label of a food, whether packaged or not, must be:
- clearly and prominently shown
- readily discernible to the purchaser or consumer under the customary conditions of purchase and use
Examples of information on a food label that may not be considered as satisfying the above mentioned legibility requirements include coloured text on a similarly coloured background, and may include impressed or embossed letters on cardboard or plastic where there is no prominence or contrast.
Unless otherwise specified in Regulations, mandatory information required on consumer prepackaged foods under the Safe Food for Canadians Act (SFCA) and SFCR must be shown in characters of at least 1.6 mm (1/16 inch) in height. The height of the characters is based on the height of an upper case letter if the words are shown in upper case only, or the height of the lower case letter "o" if the words appear in lower case or in both upper and lower case [210(1) and (2), 211, SFCR].
If a word or expression that appears in quotation marks in the SFCR is required to be shown on a label, it may, unless otherwise provided, be shown in upper or lower case, or both, so long as it meets the legibility and character height requirements. An example of this is the statement "Keep Refrigerated" / "garder réfrigéré" [209, SFCR].
Exception
For very small packages that have a principal display surface of 10 square centimetres (1.55 square inches) or less, information required by Part 11 of the SFCR, other than the numerical information in the declaration of net quantity, may appear in letter height of at least 0.8 mm (1/32 inch), provided all the information required by Division 2 of Part 11 of the SFCR appears on the principal display panel [210(3), SFCR].
Specific cases
Type size based on principal display surface
Schedule 6 of the SFCR includes the table below, which outlines the minimum character heights that must be used on the label of consumer prepackaged foods based on the area of the principal display surface for the following core labelling requirements [229, SFCR]:
- numerical information in the declaration of net quantity
- statement about imitation, artificial or simulated flavours
| Item | Area of principal display surface | Minimum character height |
|---|---|---|
| 1 | ≤ 32 cm2 (5 inches2) | 1.6 mm (1/16 inch) |
| 2 | > 32 cm2 (5 inches2) but ≤ 258 cm2 (40 inches2) | 3.2 mm (1/8 inch) |
| 3 | > 258 cm2 (40 inches2) but ≤ 645 cm2 (100 inches2) | 6.4 mm (1/4 inch) |
| 4 | > 645 cm2 (100 inches2) but ≤ 2 580 cm2 (400 inches2) | 9.5 mm (3/8 inch) |
| 5 | > 2 580 cm2 (400 inches2) | 12.7 mm (1/2 inch) |
Note that in cases where the net quantity declaration or flavouring ingredient information is shown on all or part of a display card, "Area of principal display surface" in the table above should be read as "Total area of the surface of the display card visible under customary conditions of sale or use" [229(2), SFCR].
Legibility requirements for the inspection legend
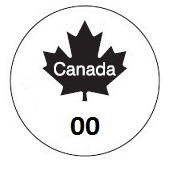
The inspection legends set out in Figures 1 and 2 of Schedule 2 of the SFCR and shown below are prescribed for the purposes of the definition of inspection mark in section 2 of SFCA [179, SFCR]. Refer to Inspection marks for more information on inspection legends.
Download high resolution image (EPS 549 kb) (When using this high resolution image, the regulated party must ensure legibility requirements are met, including minimum type size.)
Description of image: Inspection legend, Figure 1 of Schedule 2, SFCR
The inspection legend consists of the outline of a circle containing a solid, black maple leaf with the word "Canada" in white lettering centered within the maple leaf. Beneath the maple leaf, the number "00" in the inspection legend is to be replaced by the number identifying the licence holder's establishment.

Download high resolution image (EPS 560 kb) (When using this high resolution image, the regulated party must ensure legibility requirements are met, including minimum type size.)
Description of image: Inspection legend, Figure 2 of Schedule 2, SFCR
The inspection legend consists of the outline of a circle containing a solid, black maple leaf with the word "Canada" in white lettering centered within the maple leaf. The number identifying the licence holder's establishment does not appear in this legend.
Persons authorized to apply or use the inspection legend are responsible for ensuring Figures 1 and 2 of Schedule 2 of the SFCR are accurately reproduced. Figures 1 and 2 each consist of a circular outline containing a maple leaf with the word "Canada" written across the maple leaf. When Figure 1 is used, the number identifying the licence holder's establishment must replace the numbers "00" that appears beneath the maple leaf [2, 14, SFCA; 179, 183 and Schedule 2, SFCR].
All components of Figures 1 or 2 must be in the same proportions as those shown in the images set out in Schedule 2. The outline of the circle is a specific component of the figures and must be distinctly shown as a separate object from the background. The word "Canada" must be distinguishable within the maple leaf. These requirements correspond to a minimum circle diameter of the figures of about 14 mm [Schedule 2, SFCR].
When the inspection legend is used or is to be used as a label of an edible meat product, a prepackaged processed egg product or prepackaged fish, it must be clearly and prominently shown and readily discernible to the purchaser under the customary conditions of purchase and use. The size of the figures may be required to increase as the size of the package increases so that the inspection legend is prominently shown [208, SFCR]. Refer to the Inspection legend section of the Meat and poultry products page for the minimum size required when the inspection legend is applied directly to an edible meat product that is not prepackaged.
In the case of Figure 1 when it is used or is to be used as a label, the numerals "00" (that is to say, the number identifying the licence holder's establishment) must be a minimum of 1.6 mm in height. A larger character height may be required depending on the size in which the figure is shown in order to show all of its components in the same proportions as set out in the inspection legend image [185.1 and Schedule 2, SFCR].
In all cases where the inspection legend figures are applied or used, the circle outline, the maple leaf excluding the word "Canada" and, in the case of Figure 1, the licence holder's establishment number that replaces the numbers "00" must be shown in the same, single colour that contrasts with the colour of the background and the word "Canada" such that the figure is easily visible. Any colour combination may be used provided the contrast results in the image being easily visible.
For example, the circle outline, the maple leaf excluding the word "Canada" and the licence holder's establishment number may be applied in the same dark colour to a white or light coloured background or conversely, in the same white or light colour to a dark coloured background [Schedule 2, SFCR].
Care must be taken if these components of the figures are applied directly onto transparent packaging to ensure that the contrast results in the inspection legend being easily visible against the food product or empty container. If the figure is produced by applying ink to form the background and "Canada" word, the ink must be applied to a single coloured solid surface so that the circle outline, the maple leaf excluding the word "Canada" and the licence holder's establishment number result in being the same, single colour [Schedule 2, SFCR].
Legibility requirements for other specific labelling topics
For the list of ingredients, the phenylalanine statement, the food allergen source, gluten source and added sulphites and cross-contamination statements, the FDR prescribes specific technical requirements, including type height. Refer to List of ingredients and allergens for more information on these requirements.
For the Nutrition Facts table and front-of-package (FOP) nutrition symbol, the FDR prescribes specific format (graphic) and technical requirements including type height. Refer to Nutrition labelling for more information on these requirements. For more information on technical requirements for the FOP nutrition symbol, refer to How does the symbol have to be displayed on labels of prepackaged products? of the Front-of-package nutrition symbol labelling guide for industry.
In the case of some statements or claims regarding a food product, such as nutrient content claims or organic claims, the components of these statements or claims may be required to be of the same size and prominence when applied to the label of or in any advertisement for a food. For more information on the legibility requirements for statements or claims regarding a food product, see the conditions of use for the type of claim in the Industry Labelling Tool.
For example, if a manufacturer uses the claim "no sodium added" on the label or advertisement of a food product which meets the requirements for this claim as stated in table D. Sodium/Salt Related Statements and Claims in the Table of Permitted Nutrient Content Statements and Claims, incorporated by reference into the FDR, all of the words in this claim must be of the same size and prominence. It would not be acceptable if the words "no sodium" were bolded and appeared in a larger font size than the word "added". For more information on these requirements, refer to Making a nutrient content claim on food labels.
With respect to the lot code or other unique identifier, this information must be clearly and prominently shown on the label and be readily discernible under customary conditions of purchase and use [A.01.016, FDR; 208, SFCR]. If a core labelling requirement is used as the lot code, any minimum type height or legibility requirements that are prescribed for that labelling requirement still apply when it is used as the lot code [203, SFCR]. For information on the requirement for a lot code or other unique identifier for traceability purposes, consult Traceability-specific labelling requirements.
Additional legibility requirements may exist for specific labelling topics. Refer to the appropriate sections of the Industry Labelling Tool.
Location requirements
General
The label of a prepackaged food must be applied or attached in such a manner that the label is still applied or attached at the time it is sold. All or part of the label of a consumer prepackaged food must be applied to the principal display surface [B.01.004, FDR; 225, 227(1), SFCR].
Generally, commodity-specific information required by Division 3 of Part 11 of the SFCR for prepackaged foods may appear on any part of the label other than solely on the bottom of the container, unless otherwise specified. Labelling requirements of the FDR similarly apply [B.01.005(1) and (2), FDR; 245(2) and (3), SFCR].
Specific location information for some core labelling requirements is outlined in the table below. Note that the term "prepackaged" encompasses both "consumer prepackaged" and "prepackaged other than consumer prepackaged". For more information, refer to Definition of "prepackaged" and "consumer prepackaged".
| Requirement | Location on label of prepackaged food |
|---|---|
| Common name | On principal display panel B.01.006(1), FDR |
| Net quantity | Consumer prepackaged Prepackaged other than consumer prepackaged 221, 244.4, 245(2) and (3), SFCR |
| List of ingredients | On any part of the label other than solely on the bottom of the container B.01.005, B.01.008, FDR |
| Durable life date | On any panel, including the bottom if a reference is made elsewhere on the label that indicates the information is located on the bottom. Exceptions apply. See Manner of declaring on the Date marking and storage instructions page. B.01.005(1) and (4), B.01.008(1)(a), FDR |
| Name and principal place of business | On any part of the label other than solely on the bottom of the container B.01.005, FDR |
| Nutrition labelling | The Nutrition Facts table must be on one continuous surface of the available display surface (ADS). See Nutrition labelling for more information. The FOP nutrition symbol must appear on the principal display panel. For more details, refer to Where on the labels of prepackaged products does the symbol have to be displayed? of the Front-of-package nutrition symbol labelling guide for industry. B.01.005(5), B.01.350(1), B.01.451, FDR |
It is important to note that additional location requirements may exist for other labelling topics, including those for specific commodities. For more information, refer to the Industry Labelling Tool.
Grouping of mandatory information
Section B.01.008 of the FDR states that all mandatory information required by the FDR must appear grouped together on any part of the label, unless it is information which is required to be shown on the principal display panel or information which is specifically exempted from the grouping provisions stated in B.01.008(1)(a) of the FDR (such as the name and principal place of business of the responsible party, the durable life date, packaging date or expiration date information, the lot code required by B.27.005(a) of the FDR, the Nutrition Facts table, and regulated nutrient content claims and health claims). If the product is required to carry a list of ingredients, the information which is required to be grouped together on that product's label must be grouped with the list of ingredients.
Specific cases
Use of display cards as labels
In the case of a consumer prepackaged food whose container is mounted on a display card, the label may be applied to the surface of the display card that is displayed or visible under customary conditions of sale or use [228, SFCR].
Use of hang tags as labels
Generally, mandatory information that is required to be on the principal display panel (PDP) is not permitted to be located on a tag, unless the container does not have a surface that is displayed or visible under customary conditions of sale or use to which a label can be applied (for example, foil wrapped milk chocolate eggs in a mesh bag). In this case, mandatory information required on the PDP may be included on one side of a tag that is attached to the container, as this is considered to be the principal display surface [B.01.001(1), FDR; 1, SFCR].
Similarly, in the case of a container that is a wrapper or confining band so narrow in relation to the size of the food that it cannot reasonably be considered to have any surface that is displayed or visible under customary conditions of sale or use, mandatory information required to be on the PDP may be located on one side of a hang tag [1, SFCR].
Ornamental containers
In the case of ornamental containers, it is acceptable for mandatory information to be located on the bottom panel [227(2), SFCR].
Reverse side or pull-out portion of label
As a general rule, mandatory information is not considered readily discernible to a purchaser when it is on the reverse side or "outsert" or pull-out portion of the label (a folded or inserted panel under the label of a product), regardless of whether there is an instruction for the consumer such as "peel back here", or "lift this panel for more information" indicating that the consumer needs to peel the label off the container to read the reverse side. It is acceptable, however, to use the pull-out part of the label for information that is not mandatory.
Irregularly shaped packages
Mandatory information may become obscured by applying a label to an irregularly shaped package or product (for example, a square label which has taken the shape of the spherical object to which it is applied); as such, these packages may not meet the legibility and location requirements of section 208 of the SFCR and section A.01.016 of the FDR.
For containers with no definable bottom, mandatory information may appear on any panel, other than specific mandatory information that is required to be shown on the principal display panel.
Additional location requirements may exist for specific commodities. For more information, refer to the food-specific labelling requirements in the Industry Labelling Tool.
Definitions
- Bottom
The bottom is considered to be that part of a food or container which may reasonably be expected to be the surface on which the container rests when displayed for purchase. Some containers have several options as to how they can be displayed. If a food or container is labelled or printed in such a way that it may reasonably rest on any of the sides, then there is no bottom.
For example, as a frozen chicken dinner in a box may be displayed on more than one side, there is no definable bottom. In contrast, one would not reasonably expect a lemon meringue pie to be displayed on its side as it would result in damage to the product, and hence there is a definable bottom.
- Clearly and prominently shown
- Clearly and prominently shown, in respect of labelling information, means clearly visible and stands out on the label. It attracts notice or attention. A combination of factors contribute to the prominence and visibility of information on the label such as placement, contrast, colour, type size and type weight (for example, use of regular or bold type) of the information.
- Consumer prepackaged
- In respect of a food, means packaged in a container in the manner in which the food is ordinarily sold to or used or purchased by an individual, or in which the food may reasonably be expected to be obtained by an individual, without being repackaged, to be used for non-commercial purposes [1, SFCR].
- Label
As defined in the SFCA, label includes a legend, word or mark that is or is to be applied or attached to or included in, or that accompanies or is to accompany, a food commodity or a package [2, SFCA].
As defined in the Food and Drugs Act, label includes any legend, word or mark attached to, included in, belonging to or accompanying any food, drug, cosmetic, device or package [2, FDA].
- Ornamental container
An ornamental container means a container that, except on the bottom, does not have any promotional or advertising material thereon, other than a trade mark or common name and that, because of any design appearing on its surface or because of its shape or texture, appears to be a decorative ornament and is sold as both a decorative item and as the container of a food [B.01.001, FDR; 1, SFCR].
Ornamental containers must be substantial enough to be sold on their own merit (in other words, without the food). Ornamental containers are usually made of metal (for example, cookie tins), plastic or glass (for example, candy filled figurines). On the other hand, fabric-covered or embossed cardboard boxes for chocolates (for example, for Valentine's Day) are normally considered decorative rather than ornamental.
- Person
Person means an individual or an organization as defined in section 2 of the Criminal Code [2, FDA; 2, SFCA].
A person may therefore be an individual or an organization, and may include a consumer, a manufacturer, a retailer, an importer, a restaurant, any other commercial or industrial enterprise, an institution such as a school or hospital, and anyone else who sells, uses, or buys a food.
- Prepackaged
- Prepackaged, in respect of a food, means packaged in a container in the manner in which the food is ordinarily sold to or used or purchased by a person, and includes consumer prepackaged [1, SFCR].
- Principal display panel
As defined in the SFCR, principal display panel means:
- (a) in the case of a consumer prepackaged food whose container is mounted on a display card, the part of the label that is applied to one of both of the following:
- (i) all or part of the surface of the display surface
- (ii) all of part of the surface of the display card that is displayed or visible under customary conditions of sale or use
- (b) in the case of a consumer prepackaged food whose container is an ornamental container, the part of the label that is applied
- (i) to all or part of the bottom of the container
- (ii) to all or part of the principal display surface, or
- (iii) to all or part of a tag that is attached to the container
- (c) in the case of a consumer prepackaged food whose container is not described in paragraph (a) or (b), the part of the label that is applied to all or part of the principal display surface
- (d) in the case of a prepackaged food other than a consumer prepackaged food, the part of the label
- (i) that is applied or attached to all or part of the surface of the container that is displayed or visible under customary conditions of sale or use, or
- (ii) if the container does not have such a surface, that is applied to any part of the container except any part that is the bottom of the container; or
- (e) in the case of a food that is not a prepackaged food, the part of the label that is applied or attached to all or part of the surface of the food that is displayed or visible under customary conditions of sale or use [1, SFCR]
As defined in the FDR, principal display panel means, despite the meaning of principal display panel in section A.01.010,
- (a) in the case of a label that is applied to a consumer prepackaged food within the meaning of section 1 of the SFCR, the principal display panel as described in paragraphs (a) to (c) of the definition of that term in that section (see SFCR definition for principal display panel above)
- (b) in the case of a label that is applied to a prepackaged product other than a consumer prepackaged food subject to the SFCR, the part of the label that is applied to all or part of any side or surface of the container that is displayed or visible under normal or customary conditions of sale or use and, if the container does not have such a side or surface, the part of the label that is applied to any part of the container except on the bottom; or
- (c) in the case of a label that is applied to a food that is not a prepackaged product, the part of the label that is applied to all or part of the side or surface of the food that is displayed or visible under normal or customary conditions of sale or use [B.01.001(1), FDR]
- (a) in the case of a consumer prepackaged food whose container is mounted on a display card, the part of the label that is applied to one of both of the following:
- Principal display surface
The SFCR define principal display surface in respect of the container of a consumer prepackaged food. It means:
- (a) if the container has a surface that is displayed or visible under customary conditions of sale or use, the total area of that surface, excluding any surface that is the top of the container
- (b) if the container has a lid that is the part of the container that is displayed or visible under customary conditions of sale or use, the total area of the top surface of the lid
- (c) if the container does not have a particular surface that is displayed or visible under customary conditions of sale or use, 40% of the total surface area of the container, excluding any surface area that is its top and bottom, if it is possible for that 40% to be displayed or visible under customary conditions of sale or use
- (d) if the container is a bag with surfaces of equal dimensions, the total area of one of the surfaces
- (e) if the container is a bag with surfaces of different dimensions, the total area of one of the largest surfaces
- (f) despite paragraphs (a) to (e), if the container does not have a surface that is displayed or visible under customary conditions of sale or use to which a label can be applied, the total area of one side of a tag that is attached to the container
- (g) despite paragraphs (a) to (e), if the container contains wine that is exposed for sale, any part of the surface of the container, excluding its top and bottom, that can be seen without having to turn the container, and
- (h) if the container is a wrapper or confining band that is so narrow in relation to the size of the food that it cannot reasonably be considered to have any surface that is displayed or visible under customary conditions of sale or use, the total area of one side of a tag that is attached to the container [1, SFCR]
As defined in the FDR, principal display surface in respect of a prepackaged product, means:
-
- (a) if the package has a surface that is displayed or visible under customary conditions of sale or use, the total area of that surface, excluding any surface that is the top of the package
- (b) if the package has a lid that is the part of the package that is displayed or visible under customary conditions of sale or use, the total area of the top surface of the lid
- (c) if the package does not have a particular surface that is displayed or visible under customary conditions of sale or use, 40% of the total surface area of the package, excluding any surface area that is its top and bottom, if it is possible for that proportion of the total surface area to be displayed or visible under customary conditions of sale or use
- (d) if the package is a bag with surfaces of equal dimensions, the total area of one of the surfaces
- (e) if the package is a bag with surfaces of different dimensions, the total area of one of the largest surfaces
- (f) despite paragraphs (a) to (e), if the package does not have a surface that is displayed or visible under customary conditions of sale or use to which a label can be applied, the total area of one side of a tag that is attached to the package
- (g) despite paragraphs (a) to (e), if the package contains wine that is exposed for sale, any part of the surface of the package, excluding its top and bottom, that can be seen without having to turn the package, and
- (h) if the package is a wrapper or confining band that is so narrow in relation to the size of the food that it cannot reasonably be considered to have any surface that is displayed or visible under customary conditions of sale or use, the total area of one side of a tag that is attached to the package [B.01.001(1), FDR]
- Readily discernible
Discernible is a broad term that includes the requirement for the information to be perceptible, recognizable, identifiable and distinguishable on the label.
Readily discernible, in respect of labelling information, means discernible without delay or difficulty by the ordinary person under customary conditions of purchase and use of the food (for example, under the customary conditions of lighting in retail stores). Information that is readily discernible requires that the information be both easily visible and easily read (that is to say, readily legible).
"Readily discernible" is equivalent with the meaning, in French, of "facilement visibles et lisibles" in section 208 of the SFCR and "facile à apercevoir" in paragraph A.01.016(b) of the FDR.
- Date modified: