On this page
- 1.0 Background
- 2.0 Legislative authority
- 3.0 Scope of the policy
- 4.0 Overview of import requirements for plant-based feed ingredients of concern
- 4.1 Documentation requirements for all shipments
- 4.2 Import permit
- 4.3 Questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw products of concern) – without competent authority endorsement
- 4.4 Questionnaires for plant-based feed ingredients of concern subject to heat processing prior to import to Canada
- 4.4.1 Questionnaire for plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals) – with competent authority endorsement
- 4.4.2 Questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (heat processed grains or oilseeds; including plant-based meals) – without competent authority endorsement
- 4.5 Record keeping requirements
- 5.0 Specific import requirements for plant-based feed ingredients of concern
- 5.1 Import requirements for plant-based feed ingredients of concern from countries listed in appendix 3.1
- 5.2 Import requirements for plant based feed ingredients of concern from countries listed in appendix 3.2
- 5.2.1 Plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw grains or oilseeds) from countries listed in appendix 3.2
- 5.2.2 Plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals) from countries listed in appendix 3.2
- 6.0 Shipments leaving the port of export on or before April 30, 2019
- Appendix 1: list of ports declared as secondary control zones for African swine fever
- Appendix 2: products of concern with respect to African swine fever
- Appendix 3: countries of concern with respect to African swine fever
- Appendix 4: guide to documentation requirements
1.0 Background
African swine fever (ASF) is a severe viral disease affecting domestic and wild pigs. The disease originated in Africa in the 1920's, but now is spreading in Asia and Europe. The disease is responsible for serious production and economic losses through high death rates in pigs and significant trade impacts. The financial impact of the introduction of ASF into Canada is estimated at $24 billion by Canadian industry, if such an introduction were to occur.
We have strong import controls in place to prevent the import of live pigs, pork products, and by-products from countries and zones affected with ASF. However, existing provisions in the Health of Animals Regulations do not provide the authority to implement import controls for plant based-feed ingredients.
A scientific paper has been published and additional papers are in development, indicating that plant-based feed ingredients could potentially pose a risk for the transmission of viruses, including the ASF virus.
This policy document outlines the measures developed by a collaborative working group, made up of government and industry representatives.
2.0 Legislative authority
This import policy relates to animal health import requirements and does not remove any obligation of the Canadian importer to comply with the import requirements of other acts or regulations administered by us or other Government of Canada departments or agencies.
This policy falls under the legislative authority of the Health of Animals Act and Health of Animals Regulations. Subsection 27.1(2) of the Health of Animals Act gives the Minister of Agriculture and Agri-Food the authority to declare a Secondary Control Zone (SCZ) within Canada for the purposes of preventing the introduction of a disease into Canada. There are 3 key aspects to implementing a SCZ approach:
- declaring the zone(s) and naming the disease of concern (27.1(2))
- declaring the animal or thing that is capable of being affected or contaminated by the disease (27.1(3))
- prohibiting or imposing conditions on that animal or thing (27.1(4))
3.0 Scope of the policy
The declared zones are those marine ports identified in appendix 1.
For the purpose of this policy, import restrictions will be applied only to plant-based feed ingredients of concern, with those being any grains and oilseeds and their associated heat processed grains and oilseeds (including plant-based meals) imported under the end uses "livestock feed", "for further processing", "for cleaning" or any other end use where such product may be imported for direct use in livestock feed. No import controls are being implemented for products being imported for human consumption. The specific harmonized system (HS) codes impacted by this policy are listed in appendix 2.
Import controls are only being applied to products whose origin is 1 of the countries of concern with respect to ASF, which are listed in appendix 3. This list of countries includes those that are experiencing an outbreak or have experienced an outbreak of ASF within the last 5 years, and have not subsequently been recognized as free of ASF by us.
4.0 Overview of import requirements for plant-based feed ingredients of concern
4.1 Documentation requirements for all shipments
All documentation associated with the import must be completed in either English or French.
4.2 Import permit
An animal health import permit, issued by an inspector at the CFIA's Centre of Administration, is required for the importation of the select plant-based feed ingredients from countries of concern imported for 1 of the end uses of concern noted above. Specific conditions of import will be listed in the import permit. The import permit must be issued before the shipment arrives in Canada.
The permit application process allows us to assess the risks associated with the imported material. The application provides us with the required information, including the end use of the product. The import conditions, listed on the import permit, impose obligations on the importer in relation to their imported product. Importers must read and are legally required to comply with all of the conditions listed on their import permit as well as the conditions in the order that requires the permit.
Each shipment must be accompanied by a copy of the permit at the time of importation and it must be presented at the first point of entry into Canada. Permits can be issued for single entry or multiple entry (in which case the permits are valid for 1 year).
An application for a permit to import must be accompanied by information that is adequate to allow a determination that taking a plant-based feed ingredient of concern into the secondary control zone would not or would not be likely to, result in the introduction into or spread within Canada of ASF.
4.2.1. Products of concern originating from countries listed in appendix 3.1
We recognize regionalization for animal diseases for countries listed in appendix 3.1. As a result, we may recognize some regions of such countries as free of ASF, while considering other regions to be affected.
4.2.1.1 From regions recognized free of ASF within a country listed in appendix 3.1
The application for an import permit must clearly describe the country and region of origin and the product of concern. A CFIA questionnaire is not required. The import conditions in section 5.1.1 section a) will apply.
4.2.1.2 From regions not recognized free of ASF within a country listed in appendix 3.1
A completed questionnaire(s) must be submitted along with the application for an import permit, prior to importation, attesting to how the product has been processed in the country of origin or will be processed / managed once in Canada. The questionnaires referred to in sections 4.3, 4.4.1 and 4.4.2 reflect the information requirements to be met in order to allow an import permit to be issued.
- a) Plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw grains or oilseeds)
The application for an import permit must be accompanied by a completed questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (see section 4.3). The import conditions in section 5.1.2.1 will apply.
- b) Plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals)
The application for an import permit must be accompanied by a completed questionnaire for plant-based feed ingredients of concerns subject to heat processing prior to import to Canada (see section 4.4.1 and 4.4.2). The import conditions in section 5.1.2.2 section a) or b) will apply.
4.2.2. Products of concern originating from countries listed in appendix 3.2
We do not recognize regionalization for animal diseases for countries listed in appendix 3.2. Therefore we consider the entire country as affected with ASF. A completed questionnaire(s) must be submitted along with the application for an import permit, prior to importation, attesting to how the product has been processed in the country of origin or will be processed / managed once in Canada. The questionnaires referred to in sections 4.3, 4.4.1 and 4.4.2 reflect the information requirements to be met in order to allow an import permit to be issued.
- a) Plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw grains or oilseeds)
The application for an import permit must be accompanied by a completed questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (without competent authority endorsement) (see section 4.3). The import conditions in section 5.2.1 will apply.
- b) Plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals)
The application for an import permit must be accompanied by 1 of the 2 questionnaires referred to in 4.4.1 or 4.4.2. The import conditions in section 5.2.2 section a) or b) will apply.
4.3 Questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw products of concern) – without competent authority endorsement
The import permit application and questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (without competent authority endorsement) must be completed by the facility that will be processing the product once it arrives in Canada or that will be contracting with a third party to process the product once it arrives in Canada. An import permit will not be issued to a broker in this case. Where a third-party processor is engaged to process the product, the importer remains responsible for ensuring that all applicable conditions are met, including those that are to be met by the third-party processor.
This questionnaire must be completed for all plant-based feed ingredients of concern subject to mitigation measures after import to Canada.
When a questionnaire is required, the completed questionnaire must be submitted with each additional application to import.
4.4 Questionnaires for plant-based feed ingredients of concern subject to heat processing prior to import to Canada
4.4.1 Questionnaire for plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals) – with competent authority endorsement
Competent authority means any agency, authority, department, inspectorate, minister, ministry official or public or statutory person (whether autonomous or not) of any government or of any country having jurisdiction over, but not limited to, controls to protect food safety, animal health or plant health.
The questionnaire for plant-based feed ingredients of concern subject to heat processing prior to import to Canada (with competent authority endorsement) must be completed by the exporting facility and must be verified and endorsed by a representative of the competent authority in the country of origin. A questionnaire is required for each processing facility in the exporting country (if materials are coming from more than 1). An import permit may be issued to a broker in this case.
This questionnaire must be completed for plant-based meals of concern when no further processing or mitigation measures will occur in Canada after importation.
When a questionnaire is required, a completed questionnaires must be submitted with each additional application to import.
4.4.2 Questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada (heat processed grains or oilseeds; including plant-based meals) – without competent authority endorsement
The import permit application and questionnaire for plant-based feed ingredients of concern subject to mitigation measures after to import to Canada (without competent authority endorsement) must be completed by the facility that will be processing or holding the product once it arrives in Canada or that will be contracting with a third party to process or hold the product once it arrives in Canada. An import permit will not be issued to a broker in this case. Where a third-party processor is engaged to process or hold the product, the importer remains responsible for ensuring that all applicable conditions are met, including those that are to be met by the third-party processor.
This questionnaire must be completed for plant-based feed ingredients of concern subject to heat processing prior to import to Canada that will undergo further processing or mitigation measures in Canada after importation.
Completed questionnaires must be submitted with each application to import.
4.5 Record keeping requirements
The importer must keep records that accurately identifies the product imported, clearly indicates the exporting facility of the product, the date of importation, the details of treatments applied following entry into Canada (where applicable) and the details of all locations where imported product was sold or distributed. These records should be held for a minimum of 2 years and may be subject to review by a CFIA inspector.
5.0 Specific import requirements for plant-based feed ingredients of concern
5.1 Import requirements for plant-based feed ingredients of concern from countries listed in appendix 3.1
5.1.1 From regions recognized as free of ASF in countries listed in appendix 3.1
Each shipment must be accompanied by a zoosanitary export certificate endorsed by an official veterinarian of the competent authority of the country of origin or the country of export attesting that the product of concern originated in a country, region or zone recognized by Canada as free of ASF and was not commingled with product from a country, region or zone not recognized by Canada as free of ASF.
Not commingled means that the product to be exported to Canada has not been mixed with any product listed in appendix 2 of this policy originating from a country, region, or zone that is not recognized by Canada as free of ASF or with any other material that could potentially be contaminated with ASF virus. Mixing may occur intentionally or inadvertently through use of shared processing, handling or storage facilities.
5.1.2 From regions not recognized as free of ASF in countries listed in appendix 3.1
5.1.2.1 Plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw grains or oilseed)
The shipment must be shipped by the most direct and appropriate route from the point of import to the address of destination in Canada.
Bulk shipments must be transported in either single use bulk containers or containers lined with a single use, plastic liner.
Bulk shipments must be handled in such a way to prevent cross contamination.
Products must be handled in a manner in the importing facility(ies) that prevents the direct or indirect contact of untreated product with treated product. Any direct or indirect contact of untreated materials from any source will then be considered untreated and require heat treatment or a hold period.
Prior to sale or distribution, the product must undergo a heat treatment at the importing facility in a manner such that the temperature and the duration of the heat treatment is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, where the product temperature reaches at least 70°C for 30 minutes or 85°C for 5 minutes) or the product must be held at the importing facility in Canada for a period of time and at a temperature that is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, for a minimum of 20 days at 20°C or 100 days at 10°C).
Any untreated materials must be disposed of in a manner that will not result in the product entering the feed chain or being accessible to wild pigs or other animals and as per local environmental regulations.
Post-entry oversight
A CFIA inspector may go on-site at importing facilities to review records and to verify the conditions of import have been met and that the information provided in the questionnaires is accurate.
5.1.2.2 Plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals)
- a) Questionnaire with competent authority endorsement:
The product must only be imported from the exporting facility(ies) listed on the import permit.
The shipment must be shipped by the most direct and appropriate route from the point of import to the address of destination in Canada.
Bulk shipments must be transported in either single use bulk containers or containers lined with a single use, plastic liner.
Bulk shipments must be handled in such a way to prevent cross contamination.
The product must have undergone processing in the country of origin that has included a heat treatment in a manner such that the temperature and the duration of the heat treatment is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, where the product temperature reached at least 70°C for 30 minutes or 85°C for 5 minutes).
No untreated materials have been added to the processed product following the heat treatment.
Post-entry oversight
A CFIA inspector may go on-site at importing facilities to review records and to verify the conditions of import have been met.
- b) Questionnaire without competent authority endorsement:
The shipment must be shipped by the most direct and appropriate route from the point of import to the address of destination in Canada.
Bulk shipments must be transported in either single use bulk containers or containers lined with a single use, plastic liner.
Bulk shipments must be handled in such a way to prevent cross contamination.
Products must be handled in a manner in the importing facility(ies) that prevents the direct or indirect contact of untreated product with treated product. Any direct or indirect contact of untreated materials from any source will then be considered untreated and require heat treatment or a hold period.
Prior to sale or distribution, the product must:
- undergo a heat treatment at the importing facility in a manner such that the temperature and the duration of the heat treatment is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, where the product temperature reaches at least 70°C for 30 minutes or 85°C for 5 minutes); or
- be held at the importing facility in Canada for a period of time and at a temperature that is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, for a minimum of 20 days at 20°C or 100 days at 10°C)
Any untreated materials must be disposed of in a manner that will not result in the product entering the feed chain or being accessible to wild pigs or other animals and as per local environmental regulations.
Post-entry oversight
A CFIA inspector may go on-site at importing facilities to review records and to verify the conditions of import have been met and that the information provided in the questionnaires is accurate.
5.2 Import requirements for plant based feed ingredients of concern from countries listed in appendix 3.2
5.2.1 Plant-based feed ingredients of concern subject to mitigation measures after import to Canada (raw grains or oilseeds) from countries listed in appendix 3.2
The shipment must be shipped by the most direct and appropriate route from the point of import to the address of destination in Canada.
Bulk shipments must be transported in either single use bulk containers or containers lined with a single use, plastic liner.
Bulk shipments must be handled in such a way to prevent cross contamination.
Products must be handled in a manner in the importing facility(ies) that prevents the direct or indirect contact of untreated product with treated product. Any direct or indirect contact of untreated materials from any source will then be considered untreated and require heat treatment or a hold period.
Prior to sale or distribution, the product must undergo a heat treatment at the importing facility in a manner such that the temperature and the duration of the heat treatment is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, where the product temperature reaches at least 70°C for 30 minutes or 85°C for 5 minutes) or the product must be held at the importing facility in Canada for a period of time and at a temperature that is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, for a minimum of 20 days at 20°C or 100 days at 10°C).
Any untreated materials must be disposed of in a manner that will not result in the product entering the feed chain or being accessible to wild pigs or other animals and as per local environmental regulations.
Post-entry oversight
A CFIA inspector may go on-site at importing facilities to review records and to verify the conditions of import have been met and that the information provided in the questionnaires is accurate.
5.2.2 Plant-based feed ingredients of concern subject to heat processing prior to import to Canada (heat processed grains or oilseeds; including plant-based meals) from countries listed in appendix 3.2
- a) Questionnaire with competent authority endorsement:
The product must only be imported from the exporting facility(ies) listed on the import permit.
The shipment must be shipped by the most direct and appropriate route from the point of import to the address of destination in Canada.
Bulk shipments must be transported in either single use bulk containers or containers lined with a single use, plastic liner.
Bulk shipments must be handled in such a way to prevent cross contamination.
The product must have undergone processing in the country of origin that has included a heat treatment in a manner such that the temperature and the duration of the heat treatment is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, where the product temperature reached at least 70°C for 30 minutes or 85°C for 5 minutes).
No untreated materials have been added to the processed product following the heat treatment.
Post-entry oversight
A CFIA inspector may go on-site at importing facilities to review records and to verify the conditions of import have been met.
- b) Questionnaire without competent authority endorsement:
The shipment must be shipped by the most direct and appropriate route from the point of import to the address of destination in Canada.
Bulk shipments must be transported in either single use bulk containers or containers lined with a single use, plastic liner.
Bulk shipments must be handled in such a way to prevent cross contamination.
Products must be handled in a manner in the importing facility(ies) that prevents the direct or indirect contact of untreated product with treated product. Any direct or indirect contact of untreated materials from any source will then be considered untreated and require heat treatment or a hold period.
Prior to sale or distribution, the product must:
- undergo a heat treatment at the importing facility in a manner such that the temperature and the duration of the heat treatment is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, where the product temperature reaches at least 70°C for 30 minutes or 85°C for 5 minutes); or
- be held at the importing facility in Canada for a period of time and at a temperature that is adequate to ensure that the risk of transmission has been substantially mitigated on the basis of scientific standards approved by us (for example, for a minimum of 20 days at 20°C or 100 days at 10°C)
Any untreated materials must be disposed of in a manner that will not result in the product entering the feed chain or being accessible to wild pigs or other animals, and as per local environmental regulations.
Post-entry oversight
A CFIA inspector may go on-site at importing facilities to review records and to verify the conditions of import have been met and that the information provided in the questionnaires is accurate.
6.0 Shipments leaving the port of export on or before April 30, 2019
Some of the requirements listed in sections 5.1, 5.2 and 5.3 may not apply to shipments that have left the port of export before or on April 30, 2019. However, information must be supplied that allows a determination to be made that taking a plant-based feed ingredient of concern into the secondary control zone would not or would not be likely to, result in the introduction into or spread within Canada of ASF. Factors that may be considered include:
- the zoosanitary status of the country of origin
- prior assessment of the veterinary infrastructure of the state of origin by the World Organisation for Animal Health or by us
- the nature of the product
- the process and treatment applied to the product or other risk mitigation measures
- the end use and
- the potential for cross-contamination
Appendix 1: list of ports declared as secondary control zones for African swine fever
The Port of Vancouver, defined as:
the navigable waters under the jurisdiction of the Vancouver Fraser Port Authority and the real property and immovables that the Vancouver Fraser Port Authority manages, holds or occupies as set out in the letters patent published in the Supplement to the Canada Gazette, Part 1, February 27, 1999, Vol. 133, No. 9 (as amended from time to time).
The Port of Prince Rupert, defined as:
the navigable waters under the jurisdiction of the Prince Rupert Port Authority and the real property and immovables that the Prince Rupert Port Authority manages, holds or occupies as set out in the letters patent published in the Supplement to the Canada Gazette, Part 1, May 1, 1999, Vol. 133, No. 18 (as amended from time to time).
The Port of Toronto, defined as:
the navigable waters under the jurisdiction of the Toronto Port Authority and the real property and immovables that the Toronto Port Authority manages, holds or occupies as set out in the letters patent published in the Supplement to the Canada Gazette, Part 1, June 5, 1999, Vol. 133, No. 23 (as amended from time to time).
The Port of Montreal (including the terminal of Contrecoeur), defined as:
the navigable waters under the jurisdiction of the Montreal Port Authority and the real property and immovables that the Montreal Port Authority manages, holds or occupies as set out in the letters patent published in the Supplement to the Canada Gazette, Part 1, February 27, 1999, Vol. 133, No. 9 (as amended from time to time).
The Port of Quebec, defined as:
the navigable waters under the jurisdiction of the Quebec Port Authority and the real property and immovables that the Quebec Port Authority manages, holds or occupies as set out in the letters patent published in the Supplement to the Canada Gazette, Part 1, May 1, 1999, Vol. 133, No. 18 (as amended from time to time).
The Port of Halifax, defined as:
the navigable waters under the jurisdiction of the Halifax Port Authority and the real property and immovables that the Halifax Port Authority manages, holds or occupies as set out in the letters patent published in the Supplement to the Canada Gazette, Part 1, February 27, 1999, Vol. 133, No. 9 (as amended from time to time).
Appendix 2: products of concern with respect to African swine fever
Import conditions for the commodities below may be found in the Automated Import Reference System. Both organic and conventional versions of all listed commodities are products of concern with respect to African Swine Fever.
Chapter 10
- 10 01 19 Durum wheat: other
- 10 01 99 Wheat and meslin other: other
- 10 02 90 Rye: other
- 10 03 90 Barley: other
- 10 04 90 Oats: other
- 10 05 90 Maize (corn): other
- 10 06 10 0102/0103 Cereal – Rice – Grain
- 10 07 90 Grain sorghum: other
- 10 08 10 0104/0105 Cereal – Buckwheat – Grain
- 10 08 60 0084/0085 Cereal – Triticale – Grain
Chapter 11
- all commodities
Chapter 12
- 12 01 90 0110/0111 Glycine max (Soybean), grain
- 12 01 90 6000 Soybean seeds heat processed
- 12 04 00 0113/0114 Linum usitatissimum (Linseed, Flax), grain
- 12 05 10 7200 Canola seed (grain)
- 12 05 90 7200 Rape seed (grain)
- 12 06 00 Sunflower seeds
- 12 07 60 Safflower (Carthamus tinctorius) seeds
- 12 08 Flours and meals of oil seeds or oleaginous fruits, other than those of mustard
Chapter 23
- 23 02 Brans, sharps and other residues derived from cereals or leguminous plants
- 23 03 10 6213 Corn syrup process residue with filter
- 23 03 10 6214/6244 Corn gluten meal
- 23 03 10 6215/6245 Corn gluten feed
- 23 03 10 6216/6246 Corn gluten, other
- 23 03 10 6220 Rice gluten
- 23 03 10 6223/6253 Maize zein
- 23 03 10 6224 Corn protein concentrate
- 23 04 Oil-cake and other solid residues from extraction of soya-bean oil
- 23 06 oil cake and other solid residues from extraction of vegetable fats or oils:
- 23 06 10 Of cotton seeds
- 23 06 20 Of linseed
- 23 06 30 Of sunflower seeds
- 23 06 41 Of rape or colza seeds (low erucic acid)
- 23 06 49 Of rape or colza seeds (other)
- 23 06 90 Other
- 23 03 10 6217 Corn syrup process residue
- 23 09 90 6230 Modified soybean meal
- 23 09 90 6228 Acid chlorinated soybean meal
- 23 09 90 6227 Acid chlorinated canola meal
Appendix 3: countries of concern with respect to African swine fever
(Countries that have reported active cases in domestic or wild pigs within the last 5 years and have not subsequently been recognized free of the disease by us.)
Appendix 3.1 Countries of concern where we recognize regionalization for animal diseases and some regions of the country may be recognized as free of ASF
- Bulgaria
- Croatia
- Czech Republic
- Estonia
- Germany
- Greece
- Hungary
- Italy
- Latvia
- Lithuania
- Poland
- Romania
- Slovakia
- Sweden
There is a map showing the current regionalization for African swine fever in European Union (EU) member states. For member states of the EU, any region listed as an infected area and any region listed in part 1, 2, or 3 of the Annex to Commission Implementing Regulation 2021/605 is considered by us as being affected by ASF.
Appendix 3.2 Countries of concern where we do not recognize regionalization for animal diseases and the entire country is considered affected by ASF
- Albania
- Bangladesh
- Benin
- Bhutan
- Bosnia and Herzegovina
- Burkina Faso
- Burundi
- Cabo Verde
- Cambodia
- Cameroon
- Central African Republic
- Chad
- China
- Congo
- Cote D'Ivoire
- Dominican Republic
- Gambia
- Ghana
- Guinea-Bissau
- Haiti
- Hong Kong
- India
- Indonesia
- Kenya
- Laos
- Madagascar
- Malawi
- Malaysia
- Mali
- Moldova
- Mongolia
- Montenegro
- Mozambique
- Myanmar
- Namibia
- Nepal
- Nigeria
- North Korea
- Papua New Guinea
- Philippines
- Republic of Korea
- Republic of Kosovo
- Republic of North Macedonia
- Russia
- Rwanda
- Senegal
- Serbia
- Sierra Leone
- Singapore
- South Africa
- Sri Lanka
- Taiwan
- Tanzania
- Thailand
- Timor-Leste
- Togo
- Uganda
- Ukraine
- Vietnam
- Zambia
- Zimbabwe
Appendix 4: guide to documentation requirements
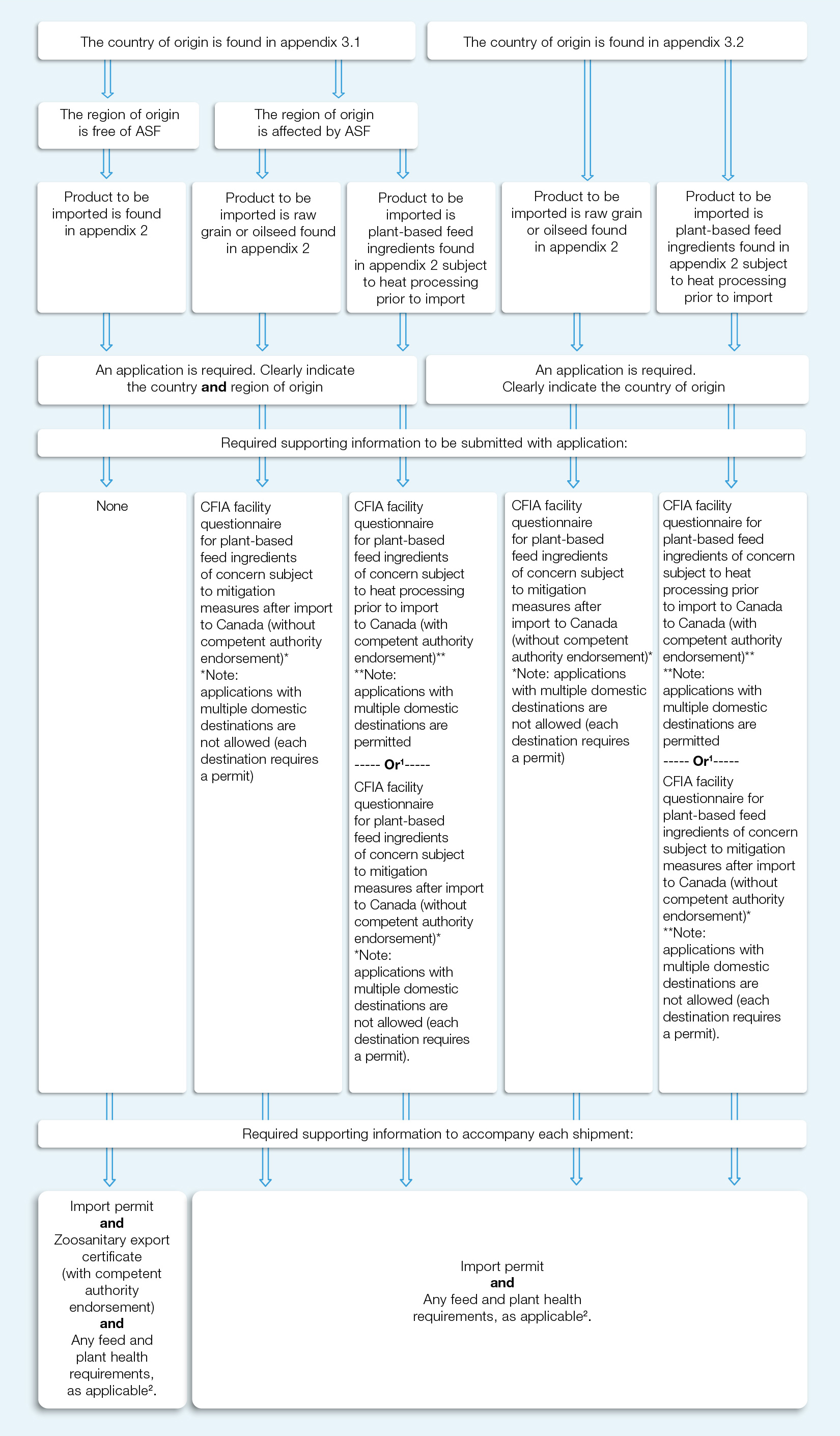
1. Refer to Section 4.4 'Questionnaires for plant-based feed ingredients of concern subject to heat processing prior to import to Canada' for details on which questionnaire may be used when the product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import
2. Importers are responsible for ensuring that the products they import into Canada comply with the requirements of all applicable Canadian legislation including requirements covered by the Health of Animals Act, Feeds Act and Plant Protection Act and their respective regulations.
Appendix 4 – Description for visually impaired.
The flow chart has a series of 6 steps, and the outcome is based on the selection made at each step.
Step 1: there are 2 options to select. Option 1 is "the country of origin is found in appendix 3.1", and option 2 is "the country of origin is found in appendix 3.2"
Step 2: If "The country of origin is found in appendix 3.1" was selected in step 1, then the following 2 options are presented: "The region of origin is free of ASF", or "the region of origin is affected by ASF".
If "The country of origin is found in appendix 3.2" was selected in step 1, step 2 is not applicable and would proceed directly to step 3.
Step 3: is comprised of 5 options, each dependent on the options chosen in the previous steps. If the option selected was "The region of origin is free of ASF" in step 2, this leads straight to the option of "Product to be imported is found in appendix 2". If the product being imported is found in appendix 2, then the result is that an application for an animal health import permit is required. The applicant must clearly indicate on the application what the country and the region of origin is in Step 4.
If the option selected was "the region of origin is affected by ASF" in step 2, then the following 2 options apply. Select either "Product to be imported is raw grain or oilseed found in appendix 2", or "Product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import".
In step 1, if the choice selected is "The country of origin is found in appendix 3.2", the option is to go directly to step 3 where the following 2 options are available, "Product to be imported is raw grain or oilseed found in appendix 2", or "Product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import".
Step 4: this step identifies if an application for an animal health import permit is required.
If the previous options selected were: the country of origin is found in appendix 3.1, the regions of origin is free from ASF, the product to be imported is found in appendix 2, and the import permit is required, then no further supporting information is required with the application submission, proceed to step 6.
If the previous options selected were the country of origin is found in appendix 3.1, the region is affected by ASF, and the product to be imported is raw grain or oilseed found in appendix 2, then an application for an animal health import permit is required. The applicant must clearly indicate the country and region of origin. Supporting information is required to be submitted with the application as described in step 5.
If the previous options selected were the country of origin is found in appendix 3.1, the region is affected by ASF, and the product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import, then an application for an animal health import permit is required. The applicant must clearly indicate the country and region of origin. Supporting information is required to be submitted with the application as described in step 5.
If the previous options selected were the country of origin is found in appendix 3.2, and the product to be imported is raw grain or oilseed found in appendix 2, then an application for an animal health import permit is required. The applicant must clearly indicate the country origin. Supporting information is required to be submitted with the application as described in step 5.
If the previous options selected were the country of origin is found in appendix 3.2, and the product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import, then an application for an animal health import permit is required. The applicant must clearly indicate the country of origin. Supporting information is required to be submitted with the application as described in step 5.
Step 5: this step outlines what information is required to be submitted to support the application for an animal health import permit.
If the previous options selected were the country of origin is found in appendix 3.1, the region is affected by ASF, and the product to be imported is raw grain or oilseed found in appendix 2, then an application for an animal health import permit is required. To support this application the applicant must supply a completed "CFIA facility questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada" (note: applications with multiple domestic destinations are not allowed (each destination requires a permit)). Step 6 describes the information that must accompany each shipment of imported product.
If the previous options selected were the country of origin is found in appendix 3.1, the region is affected by ASF, and the product to be imported is plant-based feed ingredients in appendix 2 subject to heat processing prior to import, then an application for an animal health import permit is required. To support this application the applicant must supply a completed "CFIA facility questionnaire for plant based feed ingredients of concern subject to heat processing prior to import to Canada (with competent authority endorsement)" (note: applications with multiple domestic destinations are permitted), or (note: refer to Section 4.4 'Questionnaires for plant-based feed ingredients of concern subject to heat processing prior to import to Canada' for details on which questionnaire may be used when the product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import) "CFIA facility questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada" (note: applications with multiple domestic destinations are not allowed (each destination requires a permit)). Step 6 describes the information that must accompany each shipment of imported product.
If the previous options selected were the country of origin is found in appendix 3.2, and the product to be imported is raw grain or oilseed found in appendix 2, then an application for an animal health import permit is required. To support this application the applicant must supply a completed "CFIA facility questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import to Canada" (note: applications with multiple domestic destinations are not allowed (each destination requires a permit)). Step 6 describes the information that must accompany each shipment of imported product.
If the previous options selected were the country of origin is found in appendix 3.2, and the product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import, then an application for an animal health import permit is required. To support this application the applicant must supply a completed "CFIA facility questionnaire for plant- based feed ingredients of concern subject to heat processing prior to import to Canada" (with competent authority endorsement) (note: applications with multiple domestic destinations are permitted), or (note: Refer to Section 4.4 'Questionnaires for plant-based feed ingredients of concern subject to heat processing prior to import to Canada' for details on which questionnaire may be used when the product to be imported is plant-based feed ingredients found in appendix 2 subject to heat processing prior to import) "CFIA facility questionnaire for plant-based feed ingredients of concern subject to mitigation measures after import" (note: applications with multiple domestic destinations are not allowed (each destination requires a permit)). Step 6 describes the information that must accompany each shipment of imported product.
Step 6: this step outlines what information is required to accompany each shipment of imported products.
If the previous options selected were the country of origin is found in appendix 3.1, the regions of origin is free from ASF, the product to be imported is found in appendix 2, and an animal health import permit has been acquired, then each imported shipment must be accompanied by a copy of the import permit, the Zoosanitary certificate (with the competent authority endorsement), and meet any further feed and plant health requirements as applicable. (note: importers are responsible for ensuring that the products they import into Canada comply with the requirements of all applicable Canadian legislation including requirements covered by the Health of Animals Act, Feeds Act and Plant Protection Act and their respective regulations.)
All other options selected above, will require that each shipment of imported product is to be accompanied by copies of the import permit, and meet any further feed and plant health requirements as may be applicable. (note: importers are responsible for ensuring that the products they import into Canada comply with the requirements of all applicable Canadian legislation including requirements covered by the Health of Animals Act, Feeds Act and Plant Protection Act and their respective regulations.)