On this page
The testing information summarized here describes the typical approach for bovine TB during a disease investigation. However, the actual live animal testing for an individual herd will factor in considerations such as herd size, epidemiological data and the capacity to deliver different testing methods.
There may be limited investigative actions on premises that are not believed to be infectious and contaminated by an infected animal, for instance a premises where the animal spent less than 48 hours.
Testing for bovine TB during an investigation involves both live animal and laboratory testing. The full testing process can take up to 14 weeks as the final confirmatory test (laboratory culture) can take up to 12 weeks to complete.
There may be some situations where a second herd test is required to increase the confidence that a herd is not infected. The risk factors that would require a second test would be known before testing starts and will be explained to the producer. The second test would normally be done 6 to 12 months after the first test.
Risk assessments for herds with negative histopathology results will determine if a herd may be eligible for release from quarantine before final laboratory culture results are received.
The Compensation for Destroyed Animals and Things Regulations provide for compensation for animals sent for postmortem testing or destroyed for disease eradication.
Lifeline herds
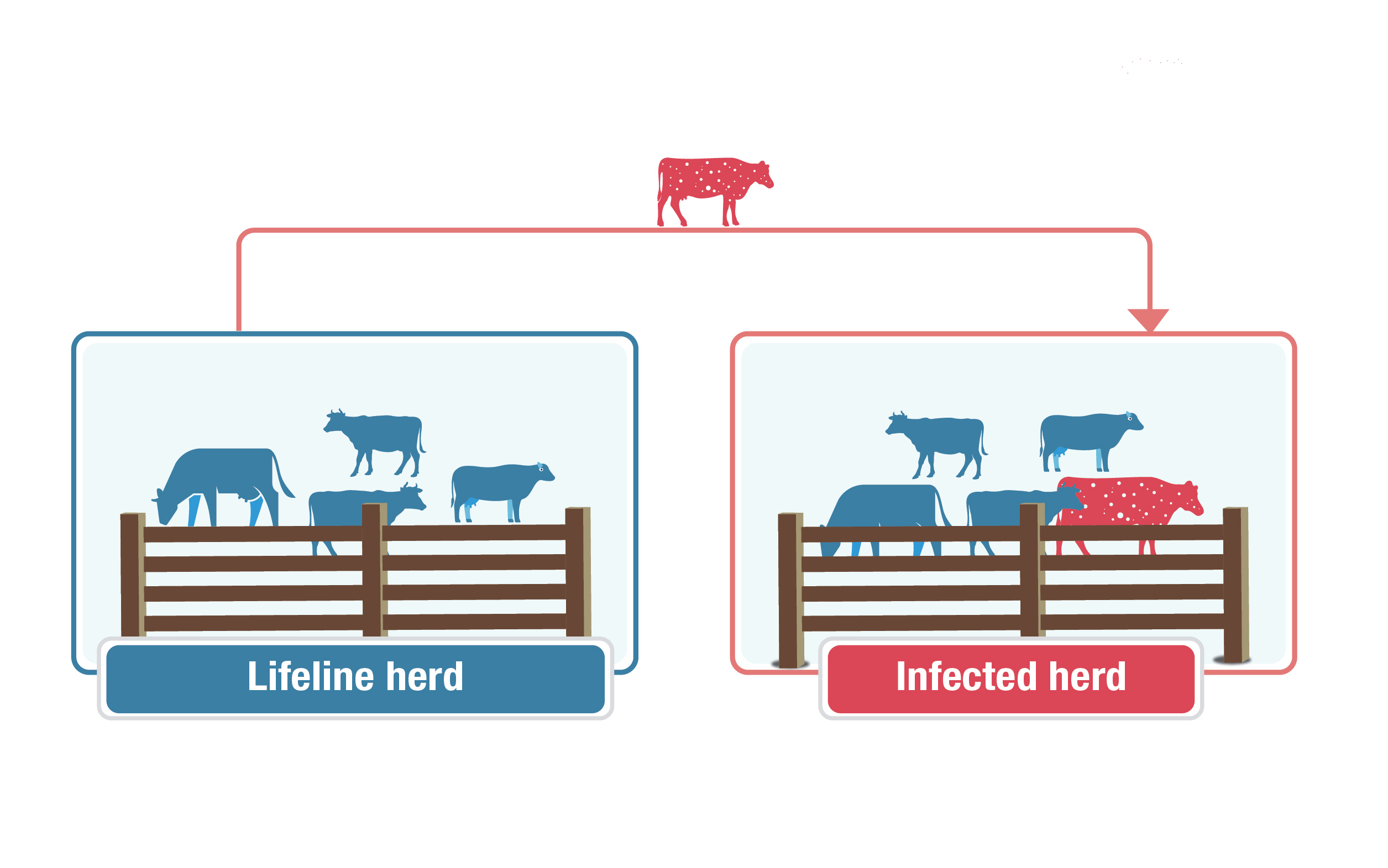
Description for lifeline herds image
This graphic explains the concept of a "lifeline herd." A herd of blue cows labelled "lifeline herd" is on the left. A second group labelled "infected herd" is on the right with a red cow with white speckles to represent an infected animal. A red line with a second red cow with white speckles points from the lifeline herd to the infected herd, showing movement of an infected animal between the herds.
When an animal is identified at slaughter as infected, its life is traced back to the birth herd. Every herd this animal was part of is a "lifeline herd".
Lifeline herd testing
Primary live animal test
- Caudal Fold Tuberculin (CFT)
Ancillary live animal test(s)
- Parallel ancillary tests (blood samples during the CFT testing process)
- Gamma interferon (IFN -g) assay [BOVIGAMTM] when operationally feasible (may be limited to specific sub-groups in a herd)
Postmortem testing
- All reactor animals from the primary test and/or ancillary testing go through postmortem inspection with tissue samples collected for confirmatory testing
Confirmatory testing
- Histopathology and culture testing is required for all reactors
- A Polymerase Chain Reaction (PCR) test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case
Infected herds
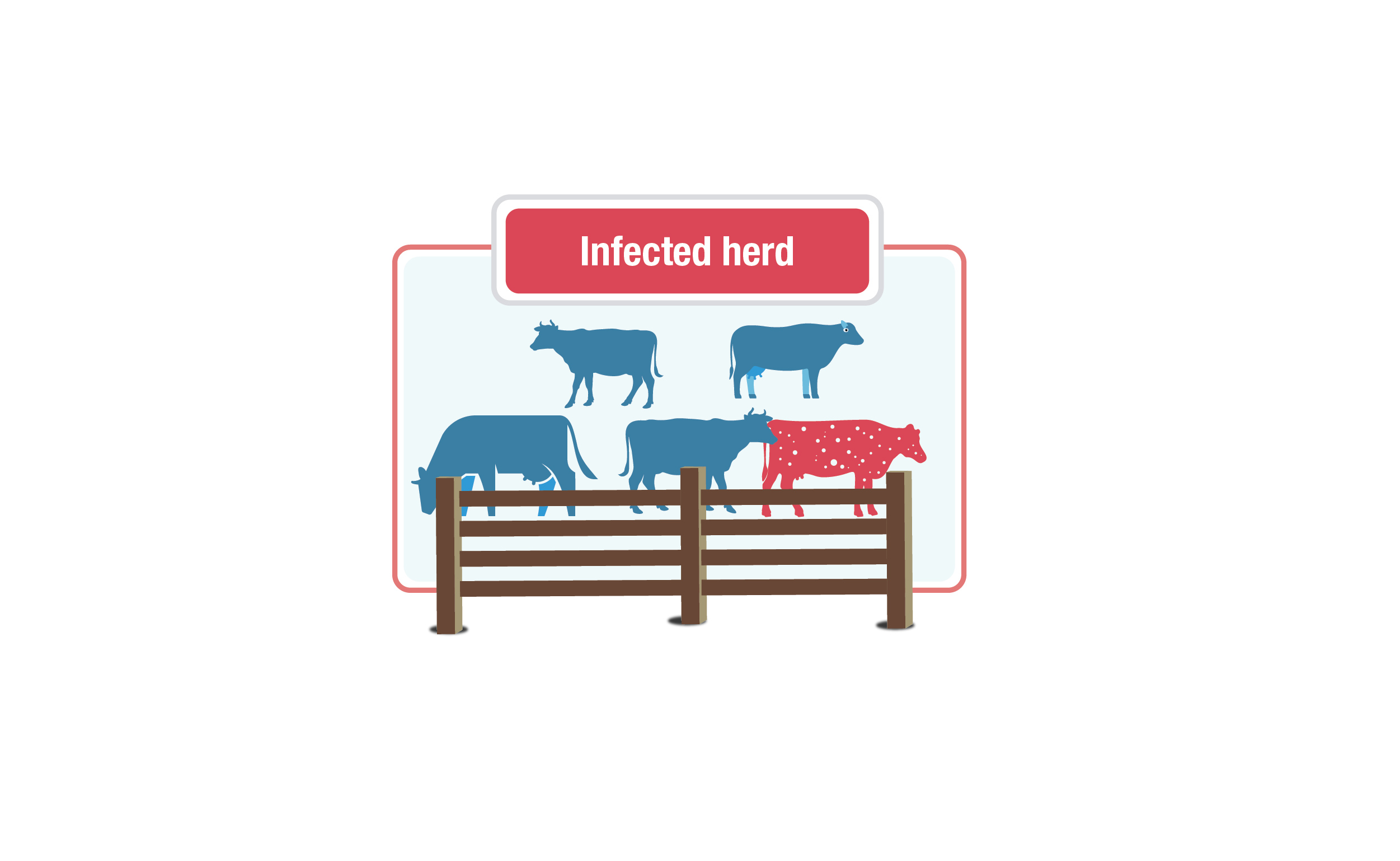
Description for infected herds image
This graphic shows a group of cows labelled "infected herd." The herd is made up of blue cows, with one red cow with white speckles to represent an infected animal within the herd.
A herd will be declared infected if:
- an animal that later tests positive is clearly traced back to that herd, and it had only recently been moved from there before slaughter or
- herd testing (from any category) in support of a bovine TB investigation detects an infected animal
An infected herd is tested to better understand the prevalence of the disease within the herd and to identify animals where tissue samples will be required even if there are no signs of bovine TB. Testing also informs disease investigation measures of other epidemiologically linked herds.
Infected herd testing
Primary live animal test
- CFT
Ancillary live animal test(s)
- No additional live animal tests are completed
Postmortem testing
- All reactor animals go through postmortem inspection with tissue samples collected for confirmatory testing
- All other animals go through postmortem inspection with tissue samples collected from any animals with signs consistent with bovine TB for confirmatory testing
Confirmatory testing
- Histopathology and culture testing is required for all reactors and any animals found with lesions consistent with bovine TB when slaughtered
- A PCR test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case.
Contact herds
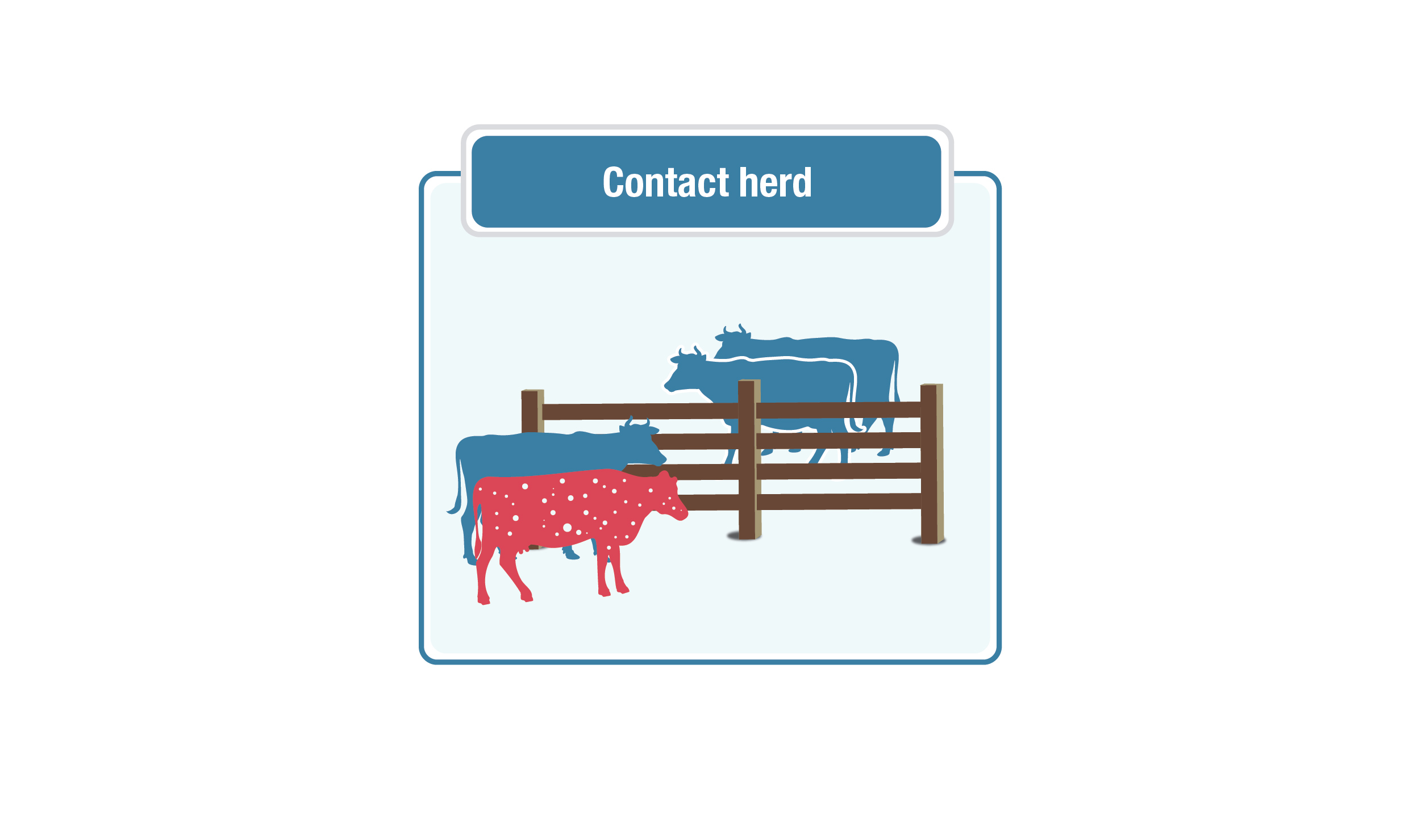
Description for contact herds image
This graphic shows a "contact herd." Two groups of cows are separated by a fence, with cows on both sides, showing potential contact between the herds. One red cow with white speckles is in one group, to represent the infected herd.
Contact herds are all herds that shared a fence line or may have co-mingled with an infected herd. Each contact herd is evaluated and assigned a disease exposure risk categorization.
High risk contact herds
A herd where there has been repeated close direct contact events with an infected herd over the critical period which equates to significant opportunities for bovine TB transmission between herds.
For example:
- commingling with an infected herd for sustained periods during summer (such as intentional shared summer pasture)
- commingling with an infected herd for any period of time during fall/winter/spring period (such as shared winter-feeding areas)
High risk contact herd testing
Primary live animal test
- CFT
Ancillary live animal test(s)
- Parallel ancillary tests (blood samples collected at the time of reading for the CFT test)
- Gamma interferon (IFN -g) assay [BOVIGAMTM] when operationally feasible (may be limited to specific sub-groups in a herd)
Postmortem testing
- All reactor animals on CFT and/or gamma interferon go through postmortem inspection with tissue samples collected for confirmatory testing.
Confirmatory testing
- Histopathology and culture testing is required for all reactors
- A PCR test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case
Moderate risk contact herds
Where there is a history of moderate direct or indirect contact events with an infected herd.
For example:
- fence-line contact with an infected herd during the fall/winter/spring period only
- commingling with an infected herd sporadically or for short periods during summer period (for example, broken fences)
- exposure to equipment that was shared with an infected herd without thorough cleaning before/after use (for example feeding or watering equipment)
- access to a water source that has been shared with an infected herd
Moderate risk contact herd testing
Primary live animal test
- CFT
Ancillary live animal test(s)
- Gamma interferon (IFN -g) assay [BOVIGAMTM] when operationally feasible (may be limited to specific sub-groups in a herd)
Postmortem testing
- All reactor animals on CFT and/or gamma interferon go through postmortem inspection with tissue samples collected for confirmatory testing
Confirmatory testing
- Histopathology and culture testing is required for all reactors
- A PCR test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case
Low risk contact herds
A herd with a history of low level direct or indirect contact events with an infected herd
For example:
- fence-line contact during summer pasture period only
- short duration contact with an infected herd
Low risk contact herd testing
Primary live animal test
- CFT
Ancillary live animal test(s)
- Serial ancillary tests for CFT reactor animals (completed after CFT)
- Comparative cervical tuberculin (CCT) test or
- Gamma interferon (IFN -g) assay [BOVIGAMTM]
Postmortem testing
- All reactor animals from the ancillary test go through postmortem inspection with tissue samples collected for confirmatory testing
Confirmatory testing
- Histopathology and culture testing is required for all reactors
- A PCR test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case
Trace out herds
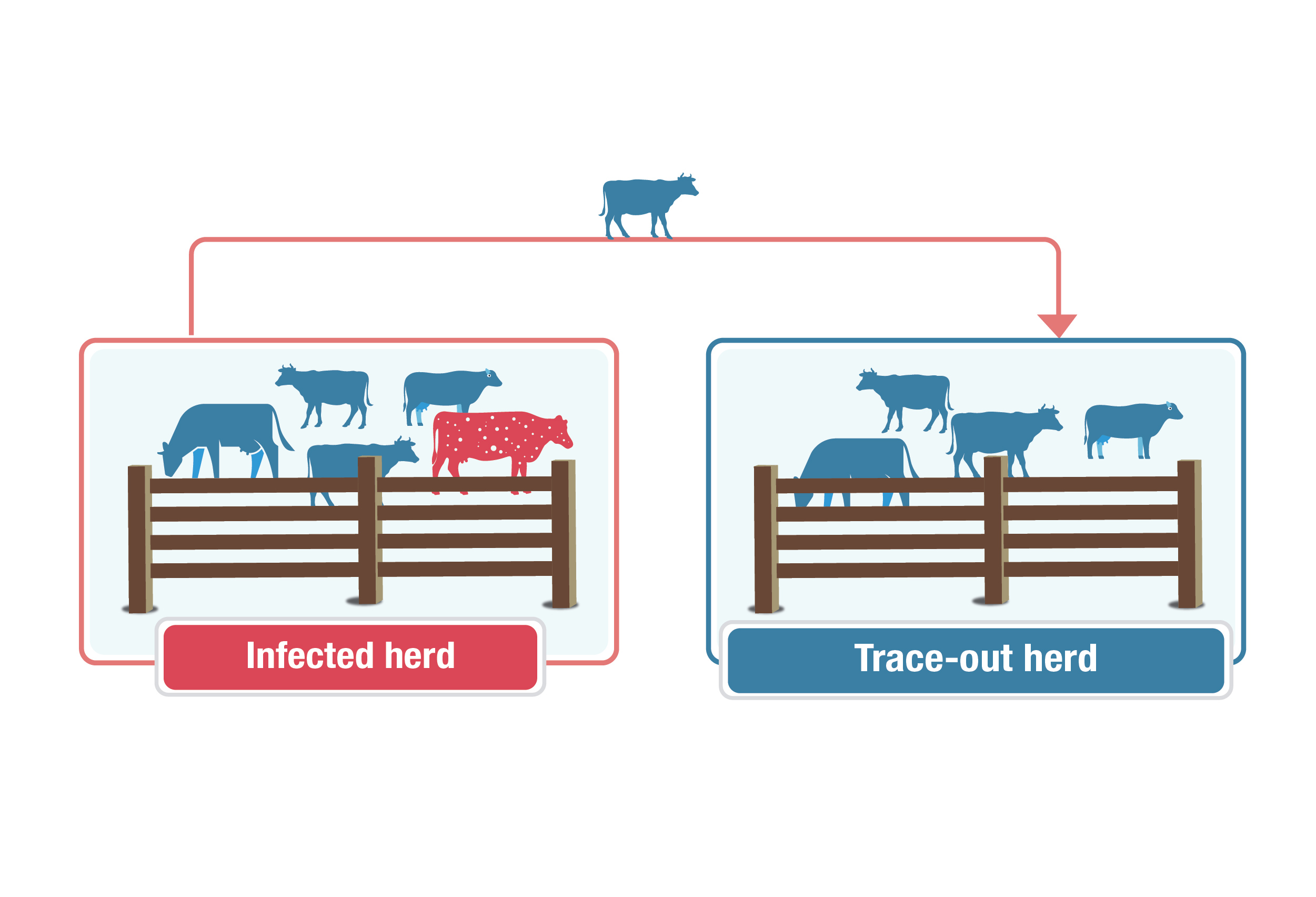
Description for trace out herds image
This graphic explains the concept of a "trace-out herd." An infected herd of blue cows, with one red cow with white speckles to represent an infected animal, is on the left. An arrow points from the infected herd to a group of all-blue cows labelled "trace-out herd," with a blue cow on the arrow showing movement of an animal from the infected herd to the trace-out herd.
All trace out herds are high risk because of their potential to spread disease.
Trace-out herds received animals from an infected herd and are therefore the highest risk for exposure to disease. All eligible animals (6 months of age or older) in the herd are tested. Any trace-out animals that are found in the herd are destroyed with confirmatory tissue testing regardless of their test results.
The same testing approach is used for
- a breeding herd where a trace out animal is located
- a breeding herd where a trace out animal has transited through the herd
- a breeding herd where the trace out animal died while in the herd, or transited through the breeding herd, and cannot be located on another premises, both resulting in the trace-out animal being unavailable for confirmatory tissue testing
Where a trace-out animal has been confirmed to have entered a breeding herd, and no evidence is available to demonstrate that it has left the herd, but the animal cannot be identified for confirmatory tissue testing, a second herd test may be performed 6 to 12 months after the first herd test.
Trace out herd testing
Primary live animal test
- CFT
Ancillary live animal test(s)
- Parallel ancillary tests (blood samples collected at the time of reading for the CFT test)
- Gamma interferon (IFN -g) assay [BOVIGAMTM] when operationally feasible (may be limited to specific sub-groups in a herd)
Postmortem testing
- All reactor animals go through postmortem inspection with tissue samples collected for confirmatory testing
- All trace-out animals are destroyed with confirmatory tissue testing regardless of their live animal test results
Confirmatory testing
- Histopathology and culture testing is required for all reactors
- A PCR test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case
Trace-out animals in feedlots
Where a trace-out animal is currently located in a terminal feedlot operation the approach involves arranging for destruction and disposal by way of slaughter in an abattoir under federal or provincial inspection.
For non-terminal feedlots, the approach will depend on whether the trace-out animal has had direct or indirect contact with animals potentially destined for a breeding herd.
Trace in herds
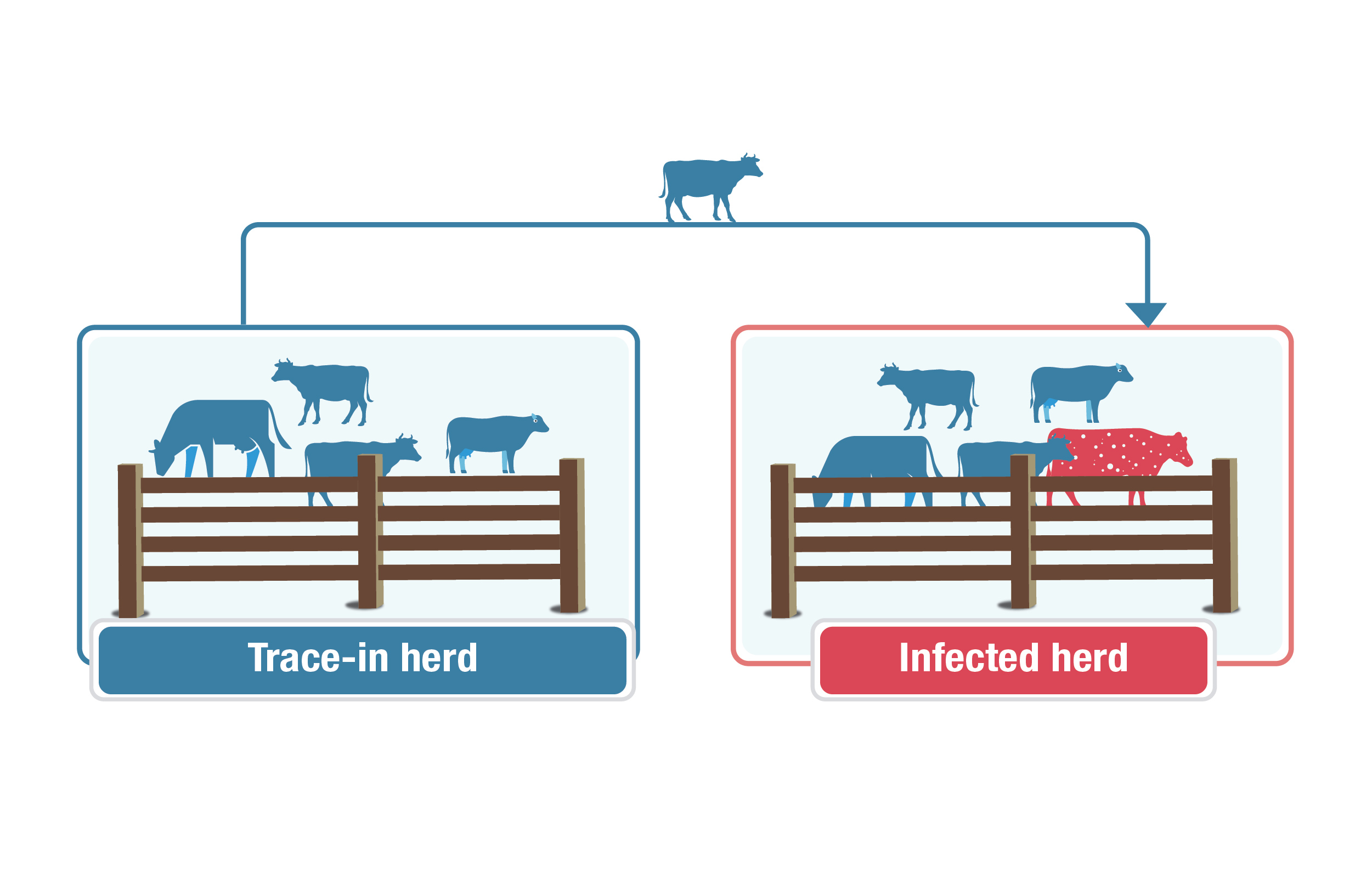
Description for trace in herds image
This graphic explains a "trace-in herd." A group of blue cows labelled "trace-in herd" is on the left, and an infected herd of blue cows with one red cow with white speckles to represent an infected animal is on the right. An arrow points from the trace-in herd to the infected herd, with a blue cow on the arrow showing movement between the herds.
Trace in herds are herds that provided animals/sold animals to an infected herd.
The standard period for identifying trace-in herds is the 5 years before the detection of bovine TB in an infected herd. Trace-in herds are tested so that a source of infection might be identified and eradicated.
For the Saskatchewan 2024 investigation, the CFIA is applying a modified trace-in period to correlate with the date when the disease was most likely introduced to the infected herd. Based on several epidemiological and disease risk elements, including genomic analysis of the bacterial cultures from those animals, the CFIA will:
- Identify trace-in animals for the 15 years prior to the 2024 confirmed infection
- Prioritize testing for herds who provided trace-in animals prior to 2021
- Based on epidemiological information, complete testing for herds who provided trace-in animals after 2021 once the priority testing is complete
Trace in herd testing
Primary live animal test
- CFT
Ancillary live animal test(s)
- Available serial ancillary tests for the testing of reactor animals
- Comparative cervical tuberculin (CCT) test or
- Gamma-interferon (IFN-ɣ) assay [BOVIGAMTM]
Postmortem testing
- All ancillary test reactor animals go through postmortem inspection with tissue samples collected for confirmatory testing
Confirmatory testing
- Histopathology and culture testing is required for all reactors
- A PCR test may also be completed on tissues where histopathology indicates the presence of mycobacteria
- A positive PCR or laboratory culture result is a confirmed case
Other testing
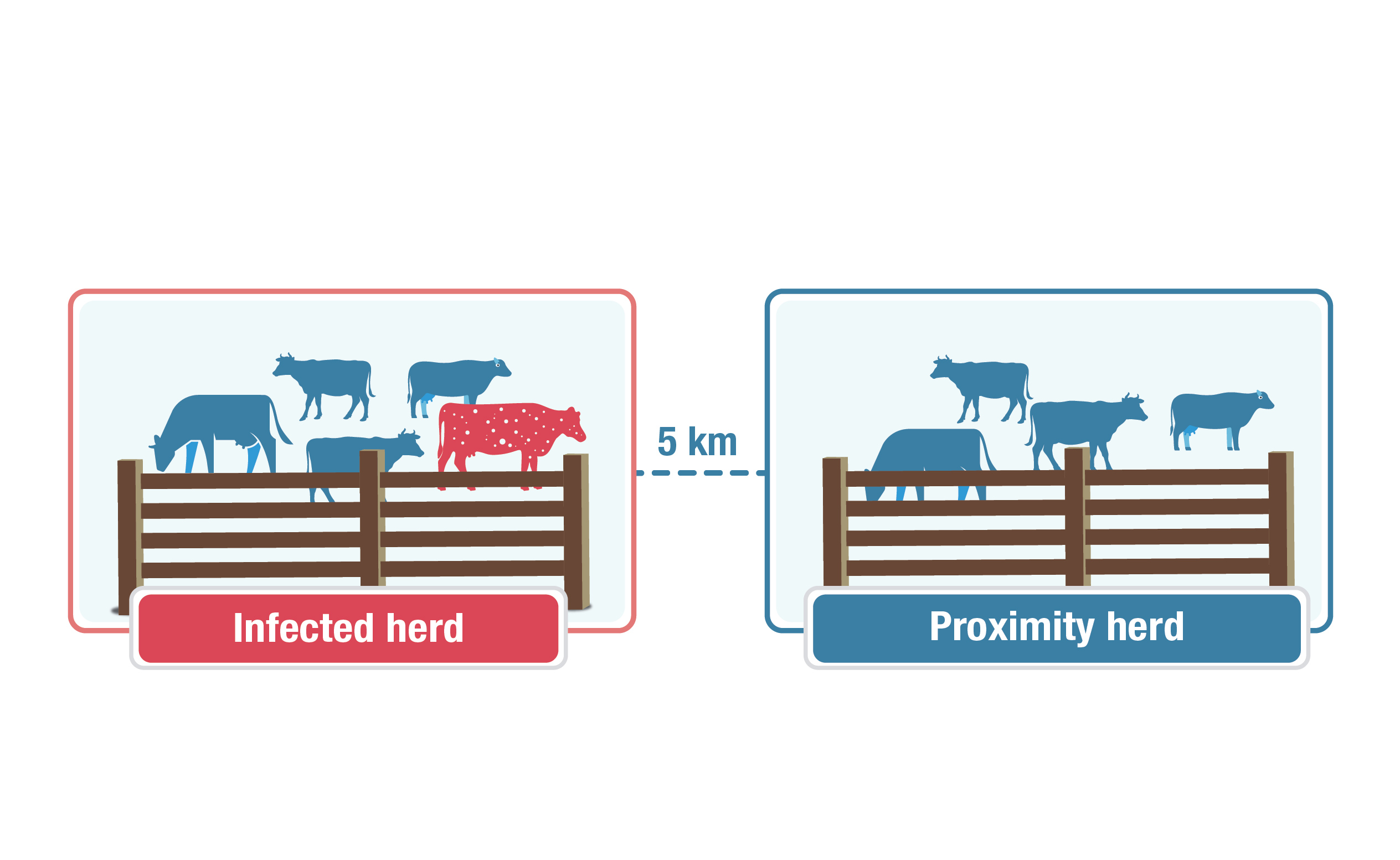
Description for other testing image
This graphic explains the concept of a "proximity herd." An infected herd with one red cow with white speckles to represent an infected animal is on the left, and a group of blue cows labelled "Proximity herd" is on the right. A dotted line with "5 km" connects the two herds, showing the distance within which a herd is considered a proximity herd.
Proximity testing of herds of susceptible livestock located in a 5 km area from the boundary of each infected premises. Testing methods will be determined by the species involved and a risk evaluation.