Date: November 21, 2019
Table of contents
- Overview of the Canadian Food Inspection Agency (CFIA)
- First 100 Days
- Hot Issues
Overview of the Canadian Food Inspection Agency
Legislative Mandate
- Develop and deliver inspection and other services to:
- Protect plant resources from pests, diseases and invasive species;
- Prevent and manage animal diseases, including diseases that threaten human health (e.g. avian influenza);
- Prevent and manage food safety risks (e.g. inspection, food recalls);
- Contribute to consumer protection (e.g. labelling claims); and
- Facilitate market access for Canada's food, plants and animals.
Division of Responsibilities between Ministers
Minister of Agriculture and Agri-food
- Responsible for:
- Agriculture and agri-food economic and trade issues
- CFIA commodity-specific legislation for plants and animals as well as non-food safety provisions of the Safe Food for Canada Act and the Food and Drugs Act (FDA)
- Plants
- Fertilizers Act
- Plant Protection Act
- Seeds Act
- Planter Breeders' Rights Act
- Animals
- Feeds Acts
- Health of Animals Act
- Food
- All non-food safety activities in the Safe Food for Canadians Act
- Food and Drugs Act
Minister of Health
- Responsible for:
- Overall direction for the CFIA
- Power to order recalls of regulated products
- Approving and tabling CFIA reports to Parliament
- Health Canada
- Sets food safety standards
- Sets food safety requirements under the Food and Drugs Act
- Conducts human health risk assessments
- CFIA
- Enforces food safety provisions of the Food and Drugs Act
- Sets inspection program requirements and enforces food safety under the Safe Food for Canadians Act
Core Responsibilities
Plant Health, Animal Health, Food Safety, International Trade
- A regulator - To set rules and verify compliance with the rules
- A risk manager - To safeguard diverse public risks related to public health, economics and environment
- A facilitator - To improve the regulatory interface with industry and trading partners
Plant Health
- Protect Canada's plant resource base
- Includes crops, horticulture, nurseries, forest resources and products, greenhouses, seeds, fertilizers, plants with novel traits, invasive alien species
- Protect Canada's plant resource base, environment and plant-related industries by:
- Preventing the introduction and spread of pests that could damage Canadian production and the income of Canadian producers;
- Verifying farmers have access to safe, effective and innovative agricultural inputs (e.g., seed, fertilizer) that support environmental sustainability;
- Fostering innovation through protection of intellectual property (i.e., plant breeders' rights); and
- Maintaining the reputation of Canadian agricultural products in the global marketplace as being high-quality, pest free and safe.
Animal Health
- Protect Canada's animal resource base and Canadians from diseases
- Includes livestock, poultry, animal feeds, vaccines and fish and seafood
- Minimize risks to Canada's terrestrial and aquatic animal resource base, and ensure the safety of animal feeds, products and vaccines by:
- Protecting Canada's animals, including aquatic animals, from diseases;
- Managing animal disease incidents and emergencies (e.g. avian influenza);
- Promoting and regulating animal welfare, in transportation and in slaughter; and
- Verifying that animal feeds and vaccines are safe and effective.
Food Safety
- Safeguard Canada's food supply
- Includes health and safety and labelling
- Shared responsibility (see Annex 1)
- Minimize health and safety risks to Canadians by:
- Protecting Canadians from preventable food safety hazards
- Managing food safety investigations and recalls effectively
- Contributes to consumer protection by:
- Verifying information provided to Canadian consumers through labels and advertising is truthful and not misleading
International Trade
- Facilitate market access for Canada's plants, animals and food
- Contribute to market access for Canadian agriculture and agri-food by:
- Influencing the development of international rules and standards for plant protection, animal health and food safety through international standard-setting bodies
- World Organization for Animal Health (WOAH; founded as Office International des Épizooties (OIE))
- Codex Alimentarius Commission (CODEX) (Food)
- International Plant Protection Convention (IPPC)
- Engaging trading partners
- Negotiating import / export conditions and technical agreements and standards
- Working in collaboration with Agriculture and Agri-Food Canada and Global Affairs Canada
- Influencing the development of international rules and standards for plant protection, animal health and food safety through international standard-setting bodies
CFIA resources (in millions)
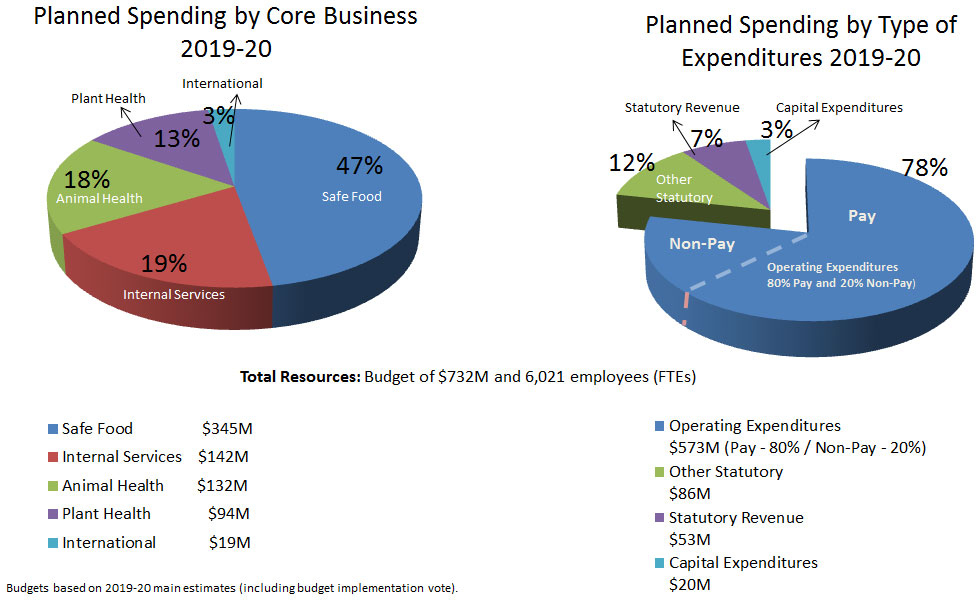
Description for CFIA resources chart
| Core Type of Expenditure | Core Planned Spending | % |
|---|---|---|
| Safe Food | $345M | 47% |
| Internal Services | $142M | 19% |
| Animal Health | $132M | 18% |
| Plant Health | $94M | 13% |
| International | $19M | 3% |
| Type of Expenditures | Core Planned Spending | % |
|---|---|---|
| Operating Expenditures | $573M | 78% |
| Other Statutory | $86M | 12% |
| Statutory Revenue | $53M | 7% |
| Capital Expenditures | $20M | 3% |
Total resources: Budget of $732 million and 6,021 employees (FTEs)
Budgets based on 2019-20 main estimates (including budget implementation vote)
CFIA National Presence
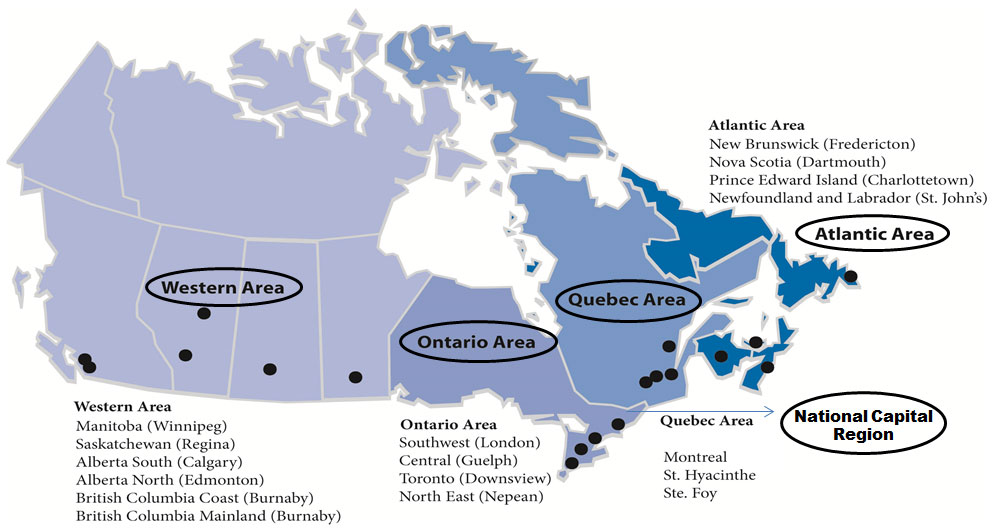
Description for CFIA National Presence chart
- Western Area
- Manitoba (Winnipeg)
- Saskatchewan (Regina)
- Alberta South (Calgary)
- Alberta North (Edmonton)
- British Columbia Coast (Burnaby)
- British Columbia Mainland (Burnaby)
- Ontario Area
- Southwest (London)
- Central (Guelph)
- Toronto (Downsview)
- North East (Nepean)
- Quebec Area
- Montreal
- St. Hyacinthe
- Ste. Foy
- Atlantic Area
- New Brunswick (Fredericton)
- Nova Scotia (Dartmouth)
- Prince Edward Island (Charlottetown)
- Newfoundland and Labrador (St. John's)
13 laboratories: Atlantic (2), Quebec (2), Ontario (3), Western (6)
CFIA Partners
International Partners
- Set import requirements, verify export requirements
- Comparability and acceptance of relevant systems (e.g. inspection)
- Develop international science-based rules, standards, etc.
Provincial, Territorial and Municipal Governments
- Enforce jurisdictional food safety, plant and animal health requirements
- Collaborate in responding to food safety incidents
- Prevent and manage plant and animal health emergencies
Federal Departments and Agencies
- Health Portfolio
- Agriculture and Agri-Food Canada Portfolio
- Global Affairs Canada
- Canada Border Services Agency
- Fisheries and Oceans Canada
- Environment and Climate Change Canada
- Natural Resources Canada
- Shared Services Canada
- Innovation, Science and Economic Development
Industry
- Production of safe food
- Comply with regulatory requirements
- Develop and implement best management practices
Consumers
- Safe food handling and preparation
- Awareness of plant and animal risks (e.g. transporting infested firewood)
Annex 1: Food Safety is a shared responsibility
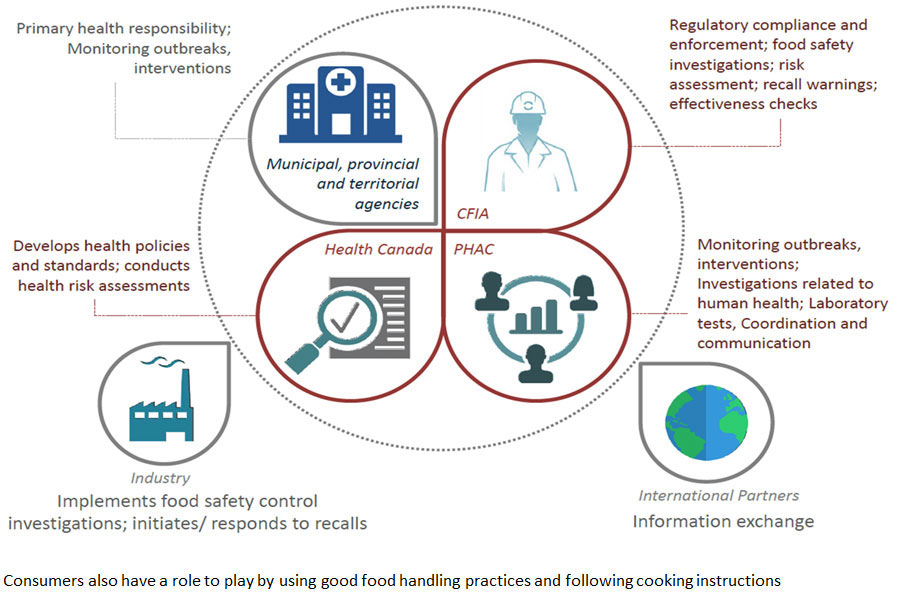
Description for Annex 1: Food Safety is a shared responsibility
- Municipal, provincial and territorial agencies
- Primary health responsibility; monitoring outbreaks, interventions
- Canadian Food Inspection Agency
- Regulatory compliance and enforcement; food safety investigations; risk assessment; recall warnings; effectiveness checks
- Health Canada
- Develops health policies and standards; conducts health risk assessments
- Public Health Agency of Canada
- Monitoring outbreaks, interventions; investigations related to human health; laboratory tests, coordination and communication.
- Industry
- Implements food safety control investigations; initiates/responds to recalls
- International Partners
- Information exchange
- Consumers also have a role to play by using good food handling practices and following cooking instructions.
Annex 2: CFIA organizational structure
Senior Executive

Siddika Mithani, President, Canadian Food Inspection Agency

France Pégeot, Executive Vice-President, Canadian Food Inspection Agency
Delivery of CFIA mandate

Jaspinder Komal, Vice-President, Science Branch
- Provides scientific advice and diagnostic and testing services.
Colleen Barnes, Vice-President, Policy and Programs
- Provides strategic policy advice and sets out program policies and procedures.
Theresa Iuliano, Vice-President, Operations Branch
- Delivers inspection programs and takes compliance and enforcement action.
Fred Gorrell, Assistant Deputy Minister, International Affairs Branch
- Leads on market access and international regulatory trade issues.
Nicole Bouchard-Steeves, Associate Vice-President, Operations
- Delivers inspection programs and takes compliance and enforcement action.
Robert Ianiro, Vice-President, Human Resources
- Enables talent identification, acquisition and mobilization.
Amanda Jane (AJ) Preece, Vice-President, Innovation, Business and Service Development and Chief Information Officer
- Delivers on major projects and priority change initiatives and enables information and information technology.
Dominique Osterrath, Vice-President, Corporate Management and Chief Financial Officer
- Provides oversight of financial management and assets and security management.
Jane Hazel, Vice-President, Communications and Public Affairs
- Delivers internal and external communication services.
Joanne Butler, Chief Audit Executive and Head of Evaluation Audit & Evaluation
- Leads internal audit and evaluation.
Merril Bawden, Chief Redress Officer, Integrity and Redress Secretariat
- Serves as the focal point for integrity and redress, including the Complaints and Appeals Office.
Kristine Allen, Executive Director and Senior General Counsel, Legal Services
- Provides legal services to the CFIA and AAFC.
CFIA Challenges and Opportunities
Operating Environment is Changing
- Increased global trade in agricultural and food products
- Government commitment to increase export targets to $75B (Budget 2017)
- Advanced pace of science and technological change
- Opportunities to capitalize on new techniques and technologies (e.g., genomics, electronic detection devices, data management, artificial intelligence)
- Escalating climate change impacts
- Impacts on movement and survival of pests and diseases/food safety hazards
- Deepening challenges towards international rules-based order
- Protectionist agendas, complex import requirements, tariffs, and/or non-tariff barriers
- Diverging industry and market/consumer demands
- Industry demanding regulatory intervention instead of adapting practices to customer demands
- Consumers seeking more information about their food
CFIA strategic priorities
- Modern Regulatory Toolkit; Integrated Risk Management; Consistent and Efficient Inspections; Digital First Tools and Services; Global Leader
- Highlights of achievement
- Safe Food for Canadians Regulations (came into force January 2019)
- Inspection planning driven by risk data and intelligence
- Increased inspector mobility with digital and data-driven inspections
- Enhanced on-line services (My CFIA)
- Influence and engage at international fora to promote Canada's interests
Path Forward
- From prescriptive regulations to outcome-based regulations.
- From reacting to emerging issues to preparing for and preventing emerging threats
- From pre-defined inspections frequency to inspection frequency informed by risk intelligence to target risks
- From reliance on paper-based and manual systems to increased use of technology.
- From adhering to outdated regulatory practices, tools and infrastructure to building an agile workforce to keep pace with changing environments and reduce unnecessary burdens.
- CFIA Forward Agenda:
- Regulatory Reform
- Innovation
- Efficiency
- African Swine Fever
Regulatory Reform
- Advance regulatory changes to more effectively manage risks, reduce burden on industry, and further public trust
- Explore fish and seafood labelling and traceability
- Modernization of agriculture inputs: fertilizer, feed, seeds
- Complete humane transportation framework and labelling modernization
- Improve safety through hatcheries and livestock traceability regulations
- Encourage industry to adapt to challenges in the current global environment
- Greater industry accountability in prevention activities – e.g. linking compensation to biosecurity and traceability practices
- Explore new regulatory oversight approaches (regulatory agility)
- Use data and information from businesses to inform CFIA inspection and target high risk areas
- Adjust the frequency of inspections of establishments implementing enhanced food safety management systems
Innovation
- [Redacted text]
- [Redacted text]
- [Redacted text]
- [Redacted text]
- [Redacted text]
- [Redacted text]
- Continued application of technology to identify risks and manage threats response
- Harnessing data/AI to better assess and manage risks – e.g. expansion of the Establishment Risk Assessment Model
- Equip inspectors with modernized inspection tools and capabilities
- Moving towards digital platform – e.g. drones/cameras
Efficiency
- Align resources with evolving risks
- Rebalance priorities to ensure sufficient focus on domestic and import activities versus supporting export growth
- Flexibility to manage resources to align with areas of highest risk
- Update service fee regime
- [Redacted text]
- Stable, sustainable resources to deliver core mandate
- New investment may be required to maintain program integrity and respond to heightened policy and service delivery expectations
- Optimizing the import system
- Implement Modernized Slaughter Inspection Program in Hog (MSIP-Hog)
- Increase inspection efficiency and enhance industry responsibility for food safety
African Swine Fever
- Highly contagious, fatal, viral disease; increasingly present globally, not yet in North America. Does not pose public health or food safety risk
- Hog/pork sector important to Canadian agriculture and economy:
- Canada third largest exporting country (~20% of world pork trade)
- Approximately $23.8 billion in total economic activity or output
- 20,600 direct jobs and 25,000 processing jobs
- ASF in Canada would have significant trade and economic impacts:
- All export markets would close immediately and could take minimum 6-9 months to reopen
- All pigs on an infected farm would be destroyed
- Compensation (maximums in regulation $2000 - $5000/pig) for animals ordered destroyed; one dense pork production region could be as high as $2B
- Unprecedented prevention and preparedness activities underway
- FPT and industry Pan-Canadian Action Plan for ASF
[Redacted text]
Roles and responsibilities of the Ministers responsible for the CFIA
When the Canadian Food Inspection Agency (CFIA) was established in 1997, its enabling legislation, the Canadian Food Inspection Agency Act, conferred upon it the status of a departmental corporation and not that of a department. Unlike a department, it has a distinct legal personality and its own specialized mandate and duties that are separate and distinct from those of the Minister responsible for CFIA. From a practical perspective, in a typical department, the powers flow from the Minister to the departmental officials whereas, in the case of CFIA, the majority of the powers are conferred directly on the CFIA or its President.
Ministerial responsibility for CFIA activities is divided between the Minister of Health and the Minister of Agriculture and Agri-Food. The Minister of Health holds all powers, duties and functions that are assigned to the Minister relating to food safety under any Act of Parliament administered and enforced by CFIA. The Minister of Health is responsible, although in an attenuated way, for actions taken by CFIA officials under legislative powers conferred on them directly. The Minister of Agriculture and Agri-Food has no responsibility for powers, duties or functions conferred directly on CFIA officials whether or not the authorities in question relate to food safety.
Minister of Health
The Minister of Health has responsibility for all departments and agencies in the Health Portfolio, including the CFIA. The Minister of Health plays a leadership role in shaping new policy, program and financial decisions, particularly with respect to managing risks related to food safety. The Minister's role is to set broad policy direction. In enacting the Canadian Food Inspection Agency Act, Parliament did not intend that the Minister be involved in or directly responsible for the administration and enforcement of the program legislation (i.e. the Acts listed in section 11 of the CFIA Act).
The Minister of Health enables CFIA to continue its work protecting Canadians from food safety risks by, for example:
- Delegating to officials the powers and duties that have been assigned to the Minister (e.g., issuing licences under the Safe Food for Canadians Act) as set out in the laws administered or enforced by the CFIA;
- Fixing the fees to be paid by industry using specified CFIA services (e.g., issuing an export certificate);
- Ordering a recall in the event that a company is unwilling or unable to recall a potentially unsafe product from the marketplace;
- Reporting to Parliament on the activities of the CFIA; and,
- Approving CFIA communication and engagement efforts related to food safety with Canadians and other stakeholders (e.g., industry associations).
Minister of Agriculture and Agri-Food
The Minister of Agriculture and Agri-Food is responsible for the Agriculture Portfolio and is the lead on promoting the economic well-being of the agriculture and agri-food sector. With respect to the CFIA, the Minister of Agriculture is responsible for the non-food safety legislation administered and enforced by the CFIA, including the facilitation of market access, animal health and plant protection and consumer protection elements of food legislation. The Minister of Agriculture enables CFIA to fulfill its non-food safety responsibilities by, for example:
- Delegating to officials the powers and duties that have been assigned to the Minister as set out in the animal health legislation (e.g., issuing veterinary biologic import permits), plant protection legislation (e.g., establishing the form of a plant import permit), and, any non-food safety legislation administered and enforced by the CFIA (e.g., Food and Drugs Act); and,
- Setting out the strategic direction to support the CFIA's market access facilitation in negotiating scientific and technical issues with foreign trading partners (e.g., conditions Canadian exporters need to meet in order to sell their agricultural and agri-food products abroad).
The unique relationship of the CFIA to the Minister of Health and the Minister of Agriculture and Agri-Food often requires a collaborative and joint approach to advancing related and dependent initiatives as they cross areas of responsibilities, e.g., market access issues involving food safety and animal health issues that can potentially impact the safety of the food supply (e.g., antimicrobial resistance).
Role of the Minister in Regulations
Most Acts of Parliament and associated regulations are administered by individual Ministers, and this responsibility can include a variety of powers, duties, and functions. Depending on the legislation (or regulations), the responsible Minister can be named in the Act itself or designated by the Governor in Council (i.e. Cabinet).
Typically, the various powers, duties and functions set out in an Act or regulations are assigned to the responsible Minister.
Decision-making authority in legislation often resides with the Minister. For example, in the Health Portfolio context, this authority encompasses a large number of possible kinds of regulatory decisions. On any given day, a large number of these decisions are made: for instance, there are over 14,000 inspections alone every year. Accordingly, based on long-standing legal precedent, the vast majority of decisions are exercised by departmental officials. This has four important advantages:
- Given the volume of regulatory decisions required, it is not practical for a Minister to personally exercise all of his or her authorities;
- The risk of perceived political interference in evidence-based decision-making is minimized;
- Many regulatory decisions are highly technical in nature and require a specialized (often scientific) expertise; and
- In the event that a decision is challenged in a court of law (subject to judicial review), the person who makes the decision may need to give evidence.
At all times, the responsible Minister retains the authority to personally make those decisions. However, the practice of allowing officials to exercise regulatory decision-making powers that are appropriate to their functions is common to all regulatory departments.
First 100 Days
Decision-Making
[Redacted text] digitization of export certificates
A. Issue
- Through Budget 2019, the Government announced an investment of $27.2M over five years, starting in 2019-20, for CFIA to fully digitize its export certification activities.
- [Redacted text]
B. Background
- Export certificates allow the Government of Canada to communicate trade information and intelligence with other governments. These certificates are a requirement to support international trade.
- There are several gaps in the systems supporting, collecting, and disseminating the documentation of certifications, largely due to the fact that the process remains paper-based.
- CFIA plans to move to fully digitized transactions between regulated parties, CFIA and its foreign counterpart authorities. Funding announced in Budget 2019 is a critical part of achieving this vision.
C. Considerations
- The current export certification system requires significant paper-based support, requiring inspectors to spend time on administrative duties that could be better spent on inspection tasks.
- Digitization will allow Canadian businesses to export their products more rapidly, which will support market diversification and long-term economic growth for the agricultural sector.
- Funding announced in Budget 2019 would equip CFIA with more timely, reliable data and reporting tools that will bolster CFIA's ability to effectively respond to possible outbreaks in Canada.
- Digitizing export certificates will also allow CFIA to mitigate challenges with the current system such as fraud, privacy, security, and traceability.
D. Next steps
- [Redacted text]
Annual regulatory modernization (ARM) bill
A. Issue
- Treasury Board Secretariat (TBS) is planning to move forward with an updated version of the Annual Regulatory Modernization (ARM) Bill to modernize Canada's regulatory framework.
- [Redacted text]
B. Background
- The ARM Bill was announced in the 2018 Fall Economic Statement as a means to remove outdated, duplicative or redundant regulatory requirements and to help Canada's regulatory framework keep pace with innovation, technological advances, shifting public policy priorities and business realities.
- Updating legislation that impedes making and amending regulations will support the Government's innovation and economic growth agenda, and will serve to modernize Canada's regulatory regimes.
- TBS is leading the ARM Bill process on behalf of all federal organizations.
- [Redacted text]
- [Redacted text]
C. Considerations
- [Redacted text]
- [Redacted text]
D. Next steps
- [Redacted text]
[Redacted text]
Ministerial Advisory Board (MAB)
A. Issue
- An opportunity exists for the Minister of Health to meet with members of the Ministerial Advisory Board within the first 100 days of the new mandate.
B. Background
- The role of the MAB is to advise the Minister of Health on matters within the responsibilities of CFIA. It may also provide advice to the Minister of Agriculture and Agri-Food on issues relating to trade, food safety, and animal and plant health.
- The MAB was created under Section 10 of the Canadian Food Inspection Agency Act and includes up to 12 members appointed by the Minister of Health. The CFIA Executive Vice President and/or President serve as ex-officio members of the MAB.
- The MAB typically meets twice a year, at CFIA headquarters. The last meeting was held on June 24, 2019.
- This is a newly constituted Board. In December 2018, eight individuals were selected and offered membership.
C. Considerations
- Although not compulsory, the MAB chairperson had called for a meeting soon after the federal election and there is a desire by the membership for a meeting before the end of the calendar year.
- An opportunity exists for the Minister to make up to three additional appointments to the MAB. [Redacted text]
D. Next steps
- CFIA, as the secretariat, will work with your office to determine a meeting date and establish an agenda to guide introduction to membership and its work.
Regulatory Reform
Final Approval (Canada Gazette Part II)
Agriculture and Agri-food Administrative Monetary Penalties Regulations (AAAMPR) – humane transportation
A. Issue
- To prepare for the coming into force of the revised Health of Animals Regulations (Transport of Animals) in February 2020, the CFIA will seek the Minister of Agriculture and Agri-Food's approval in [Redacted text] on proposed amendments to the Agriculture and Agri-Food Administrative Monetary Penalties Regulations(AAMPR).
- These amendments will enable the CFIA to continue to issue administrative monetary penalties (similar to a fine) in instances of non-compliance with the revised Health of Animals Regulations (Transport of Animals).
- [Redacted text]
B. Background
- In February 2020, amendments to modernize the Health of Animals Regulations (Transport of Animals) will come into force to better align with international standards, best industry practices, and current scientific knowledge regarding animal welfare during transportation.
- Amendments to the AAAMPR are needed to allow administrative monetary penalties (fines) to be issued in cases of non-compliance where appropriate (e.g. not complying with record-keeping requirements) with the amendments made to Part XII of the Health of Animals Regulations (Transport of Animals).
- Amendments to the AAAMPR will also address concerns from the Canadian Agricultural Review Tribunal and the Standing Joint Committee for the Scrutiny of Regulations. The concerns being addressed were first brought to the CFIA's attention in 2004. The amendments remove provisions that are considered ultra vires, and revise regulatory text for additional clarity.
- See Tab 3B for information related to the Health of Animals Regulations (Transport of Animals) that are coming into force in February, 2020.
C. Considerations
- [Redacted text]
- These amendments could potentially be contentious as there are opposing views concerning industry economic interests ([Redacted text]) and animal welfare ([Redacted text]).
- [Redacted text]
- [Redacted text]
D. Next steps
- [Redacted text]
Fertilizer Regulations
A. Issue
- [Redacted text] CFIA will seek approval from the Minister of Health and the Minister of Agriculture and Agri-Food on proposed amendments to the Fertilizer Regulations.
- The proposed amendments will introduce a risk-based approach to regulatory intervention by aligning the level of regulatory oversight with risks (e.g. product safety for humans, animals, plants and the environment).
B Background
- Fertilizers (substances containing plant nutrients) and supplements (substances other than fertilizers, intended to improve the physical condition of soils, or aid plant growth or crop yields) imported into or sold in Canada are regulated by CFIA.
- The proposal would amend product definitions and compositional criteria of fertilizer and supplement materials, bringing these up to date with current science, industry trends and international norms. It would also introduce amendments to labelling and record keeping requirements.
- The amendments to the Fertilizers Regulations will also:
- improve business competitiveness (e.g., by improving access to innovative fertilizers to help meet crop production demand from global markets);
- reduce administrative burden (e.g., by streamlining the registration process); and,
- improve the timeliness of pre-market assessments.
- The amendments were announced in the Regulatory Review Roadmap and the 2018 Fall Economic Statement as a means to help modernize Canada's regulatory framework.
- The proposed amendments were pre-published for consultation in Canada Gazette, Part I on December 8, 2018.
C. Considerations
- Industry was actively engaged in the development of the proposed amendments and is generally very supportive of the initiative.
- These amendments support innovation and growth and decrease regulatory burden through risk-based regulations and streamlined registration.
- Amendments also address concerns of the Standing Joint Committee for the Scrutiny of Regulations related to bilingual labelling and CFIA's duty under the Official Languages Act.
D. Next steps
- The final publication in Canada Gazette, Part II is anticipated for winter 2020.
Food Labelling Modernization
A. Issue
- [Redacted text] CFIA will seek approval from the Minister of Health and the Minister of Agriculture and Agri-Food on amendments to the Safe Food for Canadians Regulations and the Food and Drugs Regulations.
- These amendments will introduce changes to food labelling requirements and are expected to be generally supported by industry and consumers.
B. Background
- Domestic and international trade have changed significantly over the decades since many of the food labelling regulations were last amended.
- Consumers are increasingly seeking information to support their purchasing decisions and industry has expressed the need for flexibility in regulations to innovate.
- The proposed amendments would modernize requirements for, among other things, best before dates, food company information, and origin labelling for imported food.
- They would reduce industry burden by repealing unnecessary or duplicative food commodity-specific requirements and incorporating (by reference) certain requirements that are more subject to marketplace changes (e.g. standard container sizes).
- The proposed amendments to the Safe Food for Canadians Regulations and the Food and Drug Regulations were published in Canada Gazette, Part I in June 2019 and included a 75-day comment period.
C. Considerations
- CFIA has consulted extensively with a broad range of stakeholders, including consumers, on this proposal between 2013 and 2018. [Redacted text]
- CFIA is reviewing and addressing comments received during the Canada Gazette, Part I consultation.
D. Next Steps
- [Redacted text]
For Consultation (Canada Gazette Part I)
Feeds regulations
A. Issue
- [Redacted text] the CFIA will seek the Minister of Health and the Minister of Agriculture and Agri-Food's approvals to consult on a set of amendments to the Feeds Regulations.
- The proposed amendments would update the definition of livestock and its scope of species, the feed approval and registration process and the labelling requirements. These amendments will also provide more clarity, flexibility and transparency to regulated parties.
B. Background
- The proposed amendments would repeal and replace the Feeds Regulations, 1983 – outdated regulations which have not kept pace with changing environment (e.g. increased nutritional awareness, feed manufacturing and distribution, globalization of trade, etc.).
- The amendments to the Feeds Regulations were announced in the Regulatory Review Roadmap and the 2018 Fall Economic Statement, as a means to help modernize Canada's regulatory framework. This package has shifted timing from spring 2019 to winter 2020. Ministers of Health and of Agriculture and Agri-Food approved the Notice of Intent that was published on July 31, 2019 to inform stakeholders of this change in timing.
- Since 2012, the industry has been actively engaged and has consulted on the development of the proposal and stakeholder reaction is expected to be positive.
C. Considerations
- The regulatory proposal would align Canadian requirements with the United States' and European Union's animal feed safety standards and requirements wherever possible, to facilitate future regulatory cooperation efforts.
- Amendments will also address the Standing Joint Committee for the Scrutiny of Regulations' concerns related to bilingual labelling and CFIA authority for novel feeds (biotechnology).
- The CFIA consulted stakeholders during the development of the draft regulations. Comments have been addressed in the proposal [Redacted text]
- Additional concerns may be raised on the requirement for bilingual labels.
D. Next steps
- Anticipated pre-publication in Canada Gazette, Part I is in winter 2020.
Hatchery Regulations
A. Issue
- [Redacted text] the CFIA will seek the Minister of Health and the Minister of Agriculture and Agri-Food's approval to consult on a set of amendments to the Hatchery Regulations.
- The proposed amendments would:
- Merge three separate regulations into one under the Health of Animals Regulations;
- Incorporate by reference the disease and monitoring standards, allowing for simpler future revisions, if necessary; and
- Modernize the risk management component by requiring industry to devise its own preventative control plans, allowing for a more responsive and outcome-based approach.
B. Background
- The proposed amendments would aim to consolidate the requirements for licensing and operating poultry hatchery establishments in Canada into one part of the Health of Animals Regulations, under the Health of Animals Act.
- The amendments to the Hatchery Regulations were announced in the Regulatory Review Roadmap as a means to help modernize Canada's regulatory framework.
- Initial consultations occurred in 2003-2004. In total, four rounds of pre-consultations with stakeholders have occurred with the final round completed in 2017.
C. Considerations
- Poultry hatcheries are a critical point of potential dissemination of diseases which pose risks to human and animal health, such as Salmonella Enteritidis. These amendments strive to reduce illnesses in Canada from this pathogen.
- The proposed amendmends address a Federal/Provincial/Territorial commitment to have a nationally consistent program with requirements for supply flocks and hatcheries.
- There is broad support from stakeholders to modernize regulations that govern hatchery operators. Industry associations have indicated that: the proposed modifications are overdue; that sampling and testing requirements should be updated to include forms of Salmonella like Enteritidis; and, that the consolidation of the three regulations is appropriate. This was indicated in a letter to Minister of Health and Minister of Agriculture and Agri-Food on October 31, 2018.
D. Next Steps
- Anticipated pre-publication in Canada Gazette, Part I is in winter 2020.
Hot issues
African swine fever
A. Issue
African Swine Fever (ASF) is a contagious and fatal disease for pigs that has now spread through Asia, Africa and parts of Europe. Should ASF enter Canada, it would have a significant impact on the Canadian hog sector. Joint government-industry efforts are underway to both enhance prevention and prepare for an incursion of the disease in Canada.
B. Background
ASF is a highly contagious viral disease with no treatment or vaccine and a high mortality rate. ASF is not a food safety concern nor can it be transmitted to humans.
It can survive for prolonged periods of time in animal products and the environment. It is estimated that ASF has reduced pig production in China, the world's largest producer and consumer of pork, by as much as 50% in 2019.
To date, ASF has not been reported in North America, but as the disease continues to spread in Africa, Asia (e.g. Vietnam, South Korea, the Philippines), and parts of Europe (e.g. Belgium, Poland, Bulgaria, Serbia, Slovakia). The outbreak has resulted in pork shortages and high prices in a number of impacted countries, the magnitude of the outbreak is such that it is expected to have a disruptive impact on global agricultural markets for several years.
As the disease spreads globally, the risks of introduction into North America increases. Although Canada has an advanced biosecurity system compared to some countries where outbreaks have occurred, there are nonetheless several potential paths of introduction of ASF into Canada (e.g. international travelers or postal shipments with undeclared pork products entering Canada, contaminated animal feed).
As part of the Government of Canada's prevention efforts, CFIA has implemented the following measures, in addition to existing controls:
- import controls for select plant-based feed ingredients;
- media campaigns to increase traveler awareness and emphasize the importance of declaring meat products at the Canadian border; and
- 24 additional food, plant, and animal detector dog teams to be added at Canadian ports of entry to help prevent illegally imported meat products from entering into Canada [Redacted text].
Canada has a robust hog sector which exports 70% of its production valued at $4 billion representing live hogs and pork products. The sector creates 20,600 direct hog farm jobs and approximately 25,000 processing jobs. At any given time, there are approximately 14 million hogs in the production system. The cycle of production of a pig lasts 25 weeks.
Should ASF be detected in Canada, CFIA would begin epidemiological activities to determine the source and spread of disease. Infected premises would be locked down immediately and a zone or zones to contain the disease would be put around these premises. Once a zone(s) is in place, CFIA would control movement of animals, people, meat products, and equipment into, out of and within these zones (on infected premises, linked premises, and premises within a certain radius) to control the spread of the disease. Destruction, disposal, cleaning and disinfection would begin on infected premises and be carried out by industry and CFIA. Premises within the control zone(s) would be monitored with the goal of eventually confirming disease freedom outside the control zones.
Given the export focus of the sector, the status of access to export markets after a positive case will be critical for determining the economic health of the sector in the short and medium term. Under international guidelines and CFIA's requirements under the Health of Animals Regulations, a positive case of ASF in Canada would immediately stop Canadian hog and pork exports. This would result in a significant surplus of live hogs and pork products in the Canadian market place until international markets are re-opened.
Canada has been working on zoning agreements with other countries to reduce the period of market closures. Zoning is an internationally-recognized tool used to help manage diseases and facilitate international trade, areas outside of control zones are disease free zones where trade may resume more quickly. A zoning agreement has been secured with the United States (U.S). [Redacted text] These [Redacted text] countries account for almost 60% of the value of Canadian pork exports. Canada has also secured a zoning agreement with the European Union (EU), however, the EU only accounts for 0.2% of the value of Canadian pork exports.
However, even with a zoning agreement, it is expected that it could be a number of months until major markets re-open. As such, animal welfare concerns could quickly arise should a humane slaughter plan not be actioned to deal with the surplus of hogs. Market closure would hold financial implications for producers and processors (reduction in value of hogs, animal maintenance costs, slaughter costs, etc.).
C. Current status
AAFC and CFIA continue to engage with industry and provincial/territorial governments to prevent and prepare for a potential outbreak in Canada, including the development of a Pan-Canadian Action Plan for ASF to coordinate work being undertaken at the federal, provincial/territorial, and industry levels. As part of this work, CFIA has been leading the ASF Executive Management Board, a government-industry collaborative body put in place to increase information sharing and to provide direction on joint areas of work. CFIA and AAFC have also requested that provinces take on a leadership role in improving biosecurity on backyard farms, management of wild pigs, and planning for depopulation and disposal of surplus animals. Additionally, AAFC has been leading the Government-Industry Hog Supply Working Group, which is focused on gathering information from industry on the impacts on the hog sector supply chain an ASF outbreak in Canada.
AAFC has asked industry to carry out its own planning for the management of the hog supply during an outbreak as this will be a critical part of the recovery efforts for the sector. For example, AAFC participated in a Canadian Pork Council (CPC) board meeting in October 2019; where discussions focused on identifying effective strategies to manage excess hog supplies as a result of a prolonged border closure with considerations on how to stimulate hog production after borders reopen. Enhancing ASF crisis communication, in collaboration with CFIA and AAFC, was also discussed. CPC is also leading the Biosecurity pillar of the Pan-Canadian Action Plan for ASF, with work focused on ways to improve biosecurity at the farm level including through information sharing directly with producers on what on-farm actions can be taken to improve biosecurity and prevent the introduction and spread of ASF in Canada.
In addition, the Canadian Meat Council (CMC) is leading the Business Continuity pillar of the Action Plan, with work focused on recognition of compartmentalization to allow integrated supply chains to be recognized as disease free, similar to zoning, and allow for faster resumption of trade for products within a compartment.
D. Stakeholder view
Representatives from the whole supply chain (i.e., producers, processors, transporters) have been working closely with the federal government and provinces on planning and preparedness activities since the beginning of 2019.
Industry stakeholders requested funding for planning and preparedness activities and have want to be involved in the development of programming options in advance of an outbreak to ensure that funding would be available as soon as an outbreak occurred. Industry representatives have indicated they do not believe existing AAFC or CFIA programing (such as the Business Risk Management (BRM) suite) would provide adequate support to the sector.
E. Considerations
Producers have access to an existing suite of federal-provincial-territorial BRM programs that would provide some support in the event of an outbreak of ASF. AgriStability provides enrolled producers with some protection against severe margin declines, such as those caused by decreasing market prices and increased feed costs for retained hog inventories and has high participation among hog producers. Participation in AgriStability represents over 64% of hog producers and 95% of gross hog sales. Additionally, hog producers can withdraw funds from their AgriInvest accounts at any time, which are comprised of roughly 50%/50% producer and government contributions, to help manage initial cash flow challenges. Participation in AgriInvest represents 79% of hog producers and over 95% of gross hog sales. [Redacted text]
As a part of AAFC's work on ASF, departmental officials have undertaken efforts to review existing departmental funding mechanisms such as BRM programs and to examine possible policy and programming options to support the sector if there is an incursion. AAFC is preparing to address, in collaboration with industry and FPT partners, a number of issues should the need arise: orderly herd management and financial health of the sector, humane depopulation and disposal of surplus animals, retaining a functional domestic market, reopening international markets, and managing hogs in transit at the time of the event.
F. Next steps
- Continue to implement prevention measures, such as border enforcement of regulations on the importation of pork products and feed ingredients.
- Continue to work with provinces and industry on preparation, should ASF eventually make its way to Canada.
- Options are being developed for additional preparedness and prevention measures for consideration.
Humane transportation
A. Issue
- The Minister of Agriculture could hear a range of feedback from stakeholders on changes CFIA is introducing to Part XII (Transport of Animals) to the Health of Animals Regulations (HAR), which will come into force in February 2020.
- The cattle industry has raised concerns and animal welfare groups are of the view that the regulations do not go far enough.
- [Redacted text]
B. Background
- The CFIA has the regulatory authority, under the HAR, for the humane transportation of all animals transported into, out of, or within Canada.
- Following over a decade of consultations with stakeholders, CFIA published amendments to Part XII (Transport of Animals) of HAR on February 20, 2019, with a coming into force date of February 20, 2020.
- The HAR amendments modernize animal transport regulations to better align with international standards, best industry practices, and current scientific knowledge regarding animal welfare during transportation.
- [Redacted text]
C. Consideration
- There are divergent views on the issue: consumers are demanding meat products from animals that are well treated; animal advocacy groups ([Redacted text] feel the amendments have not gone far enough; and industry has expressed concerns related to the breadth of the new requirements and readiness to implement and associated costs.
- The delayed coming into force date of the regulations (February 20, 2020) was intended to provide industry time to adjust certain long-standing transportation practices. [Redacted text]
- [Redacted text]
D. Next steps
- CFIA is currently working to prepare relevant guidance for stakeholders in advance of February 2020. The guidance will help regulated parties understand and comply with the amended regulations.
- Early briefings will be scheduled with your office to discuss this evolving issue [Redacted text]
Licence suspension
A. Issue
- The CFIA has suspended the licences of a large meat processing facility in Ontario because the companies failed to comply with the Safe Food for Canadians Regulations.
- This course of action could lead to the closure of a meat processing establishment and employee lay-offs. It could also affect access to abattoirs in the Ontario region and disrupt the supply and availability of kosher meat products in Ontario.
- The issue is currently evolving and the owner and/or cattle producers may contact Ministers to discuss.
B. Background
- On September 13, 2019, CFIA inspection staff at Ryding-Regency Meat Packers (Establishment 99) located in Toronto, confirmed that the operator had not reported positive E. coli results in one of the largest producers of kosher products in Ontario. The establishment only presented negative results for CFIA review.
- E. coli O157:H7 can cause nausea, vomiting, abdominal cramps and bloody diarrhea. In severe cases of illness, some people may have seizures or strokes, need blood transfusion and kidney dialysis, live with permanent kidney damage or may die. To date, no human illnesses have been associated with implicated products from these facilities.
- A food safety investigation was launched, which led to the suspension of the licences of two establishments under the same ownership and management. Voluntary recalls were conducted by both establishments, which in turn led to additional recalls by receiving clients of the implicated products (both domestically and internationally), including at the hotel, restaurant, institutional and consumer level. Countries who received the recalled products have either been notified via the CFIA's regular process, or through the International Food Safety Authorities Network.
- On October 22nd, 2019, the CFIA met with the company representatives and presented three letters of intent to cancel for each of the three licences.
- The issuance of a Notice of Intent is in accordance with the provisions of the Safe Food for Canadians Regulations, which provides that the licence holder is to be notified of the ground for the cancellation, and to be provided an opportunity to be heard regarding the cancellation.
- [Redacted text]
C. Consideration
- There are currently no reported illnesses associated with this issue.
- Establishment 99 is one out of eight federally registered kosher meat plants in Canada eligible for ritual slaughter. As not all licensed operators choose to produce kosher meat products full-time, the licence suspension or cancellation for Establishment 99 may impact kosher meat availability and slaughter capacity generally in Ontario.
- Impacted products were distributed domestically and internationally to China, the United Arab Emirates (UAE), Saudi Arabia and Indonesia.
- On October 16th, 2019, the USDA FSIS issued a public health alert for beef products derived from imported beef from Ontario, Canada that has been recalled by Ryding Regency Meat Packers Ltd.
D. Next steps
- [Redacted text]
- CFIA is continuing the food safety investigation and will issue food recall warnings if other affected products are identified.
Bovine Spongiform Encephalopathy (BSE), submission to the World Organization for Animal Health
A. Issue
- Canada will be submitting an application to the World Health Organization for Animal Health (WOAH) in [Redacted text] to demonstrate that Canada's programs to control Bovine Spongiform Encephalopathy (BSE) meet certain risk status requirements.
- If successful, Canada's disease risk status would change from "controlled" to "negligible".
B. Background
- BSE is a progressive, fatal disease of the nervous system of cattle that is associated with the presence of an abnormal protein called a prion.
- When Canada discovered its first domestic case of BSE in 2003, borders were immediately closed for Canadian cattle, sheep, goats, and bison, as well as products from these animals. Program initiatives were put in place to manage risks to human health, animal health, and market access.
- The WOAH evaluates countries and assigns them one of three categories of risk for BSE: negligible, controlled or undetermined. A country can be categorized as having a "negligible" BSE risk if it has never had a case of BSE in a domestic animal, or if any infected domestic animals were born more than 11 years ago.
- Canada currently has "controlled" status, but is now eligible to apply for "negligible" status, which would better position Canada to access new potential markets for beef and beef products.
- Canada plans to submit its application in [Redacted text] and could be granted "negligible" status in May 2021.
C. Considerations
- To achieve "negligible" status, Canada must demonstrate that Canada's BSE controls are being effectively implemented and supported by 8 years of retrospective data. [Redacted text]
- [Redacted text] date was selected to ensure that Canada develops the most robust submission possible. CFIA and CCA have been working together and meeting on a regular basis to advance the submission.The Conservative party also announced a platform commitment to apply for BSE negligible risk status in 2020.
- CFIA is working collaboratively with stakeholders to collect data and information to submit an application to the WOAH that addresses the high standard of requirements needed to obtain "negligible" risk status. However, there is no guarantee that the status will be granted by the WOAH.
- [Redacted text]
D. Next steps
- CFIA will continue to work with industry on the collection of data and preparation of the submission.
Fish and seafood misrepresentation (food fraud)
A. Issue
- The prevalence of fish and seafood fraud in Canada has been highlighted in reports from the Standing Committee on Fisheries and Oceans and non-government organizations, such as Oceana Canada. Fish substitution, misrepresentation and food fraud in general, is an increasing global concern and an area of focus for CFIA.
B. Background
- Food fraud is the deliberate misrepresentation of food for economic gain, which may have health and safety impacts and may affect the reputations of industry and regulators. CFIA received funding as part of Budget 2019 to enhance its capacity to detect food fraud and take enforcement against it.
- Oceana Canada recently published reports indicating widespread fish misrepresentation in Canada's retailers and restaurants. Their report recommends improving traceability from the point of harvest to the point of sale as a key step towards preventing fraud and protecting vulnerable natural resources.
- CFIA is addressing fish and seafood fraud through targeted inspection and surveillance, reviewing and updating labelling requirements, and enhanced inspection and investigation capacity.
C. Considerations
- Oceana's interest in addressing fish fraud at the point of harvest is driven by environmental and ocean sustainability concerns. Fisheries management, including illegal, unreported and unregulated fishing (IUU), is the responsibility of the Department of Fisheries and Oceans.
- Provinces and Territories provide oversight at restaurants, where the highest proportion of misrepresentation has been found.
- [Redacted text]
- [Redacted text]
D. Next steps
- [Redacted text] CFIA will be developing options and recommending concrete measures to reduce the incidence of fish fraud in Canada.