On this page
Introduction
The Canadian Food Inspection Agency (CFIA) is committed to providing a clear regulatory framework for products of agricultural biotechnology, while maintaining a science-based approach that upholds Canada's high standards for safety. CFIA regulates novel seed under the authorities in Part V of the Seeds Regulations, and provides plain-language guidance that explains how the regulations are applied. CFIA is updating its guidance to help plant developers understand whether their plant lines are novel. Updated guidance will help the CFIA regulatory programs for agricultural biotechnology keep pace with new technologies like gene editing.
As part of the CFIA's commitment to science-based regulation and transparency about the regulatory process, the CFIA sought feedback on draft guidance. This report summarizes the feedback that the CFIA received from stakeholders during the consultation.
About the consultation
The CFIA sought feedback on the draft guidance from May 19, 2021 to September 16, 2021 (120 days). Consultation materials included a consultation survey, summary, and the draft guidance. Feedback from this consultation helped the CFIA determine that the draft guidance required further revisions to provide stakeholders with the clarity and predictability they need.
Who we heard from
The CFIA received 316 completed surveys, 143 emails, and 49 formal letters from:
- the Canadian public
- public and private plant developers including academia, government, and industry
- agriculture industry members including associations representing the seed and grain value chain
- not-for-profit organizations including associations representing Canadian farmers
What we heard
Feedback highlights
- in terms of which plants are regulated under Part V, CFIA heard 2 main points of view:
- conventionally bred plants and plants that could have been developed using conventional breeding, including herbicide tolerant plants, should be exempt from Part V
- all gene-edited plants should be subject to Part V
- CFIA should maintain focus on environmental safety in light of new technologies like gene editing
- CFIA should ensure Canadian growers have access to world class seed innovations, without disruption to international trade
- There is a need for improved transparency so that producers and consumers can make informed choices regarding gene-edited seeds and food
Respondents provided a range of perspectives on the draft guidance. Overall, respondents expressed views that can be summarized under 4 key themes:
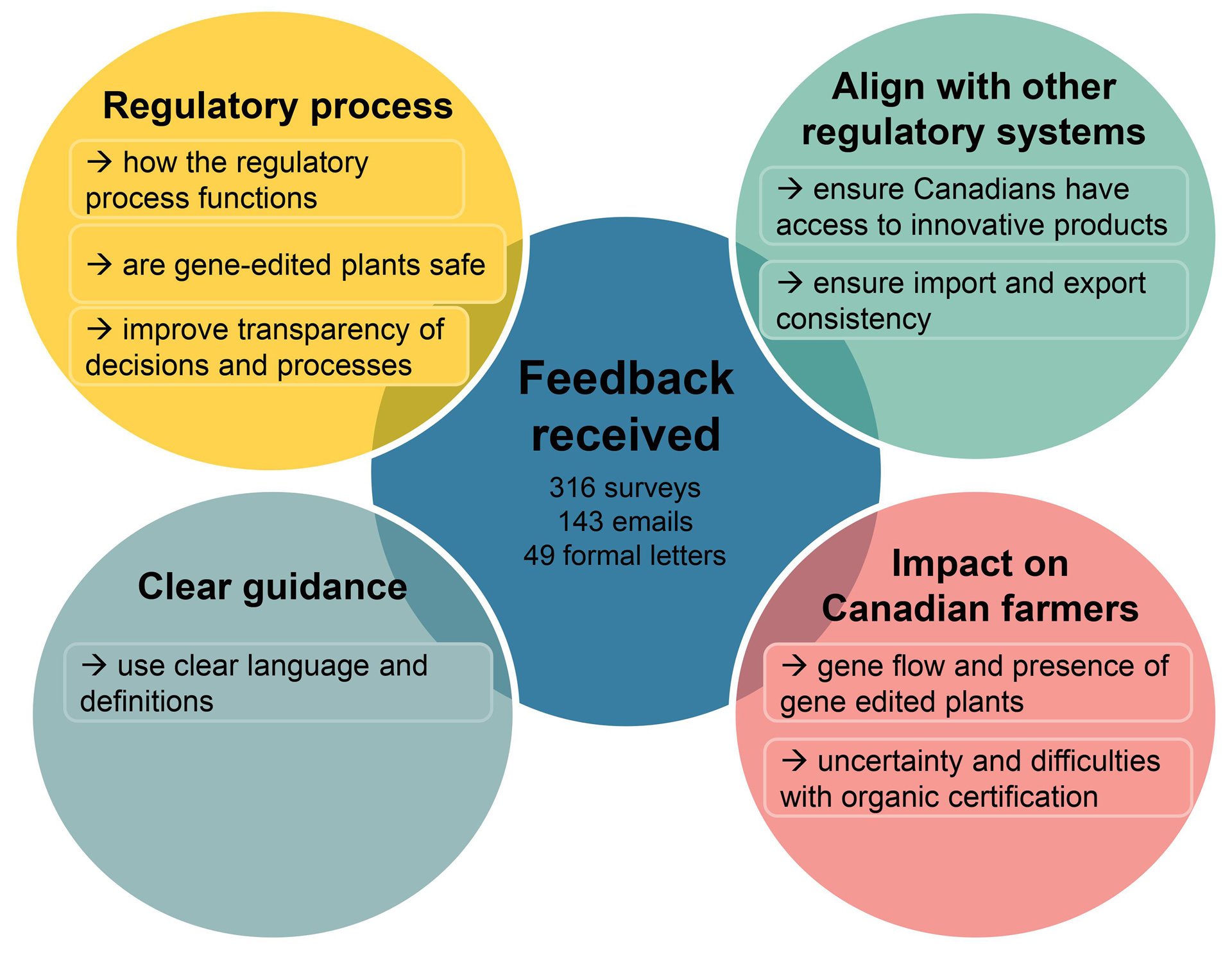
Description for Figure 1 - Diagram of key feedback themes and the related common comments or concerns
Feedback received by the Canadian Food Inspection Agency included 316 survey submissions, 143 emails, and 49 formal letters summarized into 4 key themes.
- regulatory process
- how the regulatory process functions
- are gene-edited plants safe
- improve transparency of decisions and processes
- align with other regulatory systems
- ensure Canadians have access to innovative products
- ensure import and export consistency
- clear guidance
- use clear language and definitions
- impact on Canadian farmers
- gene flow and presence of gene-edited plants
- uncertainty and difficulties with organic certification
1. Regulatory process
How the regulatory process functions
Respondents communicated varying opinions on the CFIA's overall regulatory approach, application process, assessment process, and the authorization of plants under Part V. Some of these comments were outside of the scope of an update to guidance, and would require regulatory changes to implement. In summary, some respondents indicated that the CFIA should require a mandatory assessment of all products of gene editing. Others highlighted that many of the responsibilities relating to the use of biotechnology can be well-managed by the agricultural sector. Specifically, respondents expressed comments on:
- the overall regulatory approach
- CFIA should maintain a science and product-based regulatory approach
- a need for a predictable regulatory path for products in the early-stages of development, to help guide investment decisions
- shift emphasis from what is captured to what is exempted, by clarifying existing exemptions from Part V in the guidance, rather than use the 4 plant breeding outcomes to outline what is captured
- concerns that the 4 outcomes identified in the CFIA's draft guidance were overly broad and would capture products without environmental risk
- concerns that fewer plant products of biotechnology will be assessed under the draft guidance compared to the current regulatory guidance, particularly if developers are responsible for determining whether a product may present any environmental risk
- the application process
- there should be a single application process in Canada where applicants would apply to a single organization to obtain environmental release, animal feed, and food use authorizations rather than submitting 3 separate applications
- developers should not be able to determine for themselves that a product qualifies for an exemption from Part V
- the CFIA should support developers in determining for themselves which plants are subject to Part V by providing clearer guidance
- the assessment process
- the CFIA should require notification and assessment for all gene-edited and genetically modified plants, including assessment of off-target effects
- the CFIA should limit considerations around environmental risk to natural environments, since management decisions for agricultural environments are the responsibility of the producer and other organizations (such as the Pest Management Regulatory Agency (PMRA) for pesticide labels)
- implement multi-party, independent environmental impact assessments for all gene-edited plants, including impacts on other producer groups, sustainable agriculture, and Indigenous cultural heritage
- establish a process for seeking public opinions of assessments and research trials
- apply a precautionary approach that favours holistic assessment, including impact on biodiversity, community, and economy
- establish service standards, update information requirements, and introduce assessment tiers for plants requiring assessment
- authorizations
- ban the cultivation of all gene-edited and genetically modified plants of agricultural biotechnology, including horticultural crops and forest trees
- strengthen the CFIA's authority for managing cases of non-compliance, unintentional release, and unintended effects post-authorization
- recognize the roles of Canadian producers, extension, and agricultural associations to effectively manage on-farm stewardship, particularly when the PMRA plays a major role in upholding the safe and responsible use of herbicides and pesticides
- transparency on the publication process for regulatory decisions
- the publication of information should be voluntary
- the CFIA should ensure mandatory participation in transparency initiatives, and require the publication of all such information
- the CFIA should post all:
- exemption opinions, decisions, and rationales
- active submissions for environmental safety assessments, and associated data, including confidential information
- regulatory decisions to authorize a plant, along with the details about the assessment and any stewardship plans
- information about the commercialization status of authorized plants
- the CFIA should ensure the traceability of gene-edited plants
- gene-edited seeds and foods should be subject to mandatory labelling so that producers and consumers can make informed choices
- the CFIA should publicly maintain a list of all lobbyist activities
Safety of gene editing and edited plantsFootnote 1
Several respondents expressed concerns over the release of gene-edited seeds and the potential negative impact on human and environmental health. Others commented that the guidance sufficiently recognizes the safety of gene editing. These comments included:
- strong endorsement that gene editing does not present any unique safety concerns beyond those of conventional plant breeding
- that plant products comparable to conventionally bred outcomes should qualify for exemption
- strong endorsement for evidence-based conclusions, and the effect this has on the development of this guidance
- concerns about:
- the unknown impacts of gene editing on the nutritional value of food
- herbicide and pesticide use, including:
- requests to ban the use of herbicides and pesticides,
- calls to ban herbicide-tolerant plants that would encourage more pesticide use
- whether off-target edits can be accurately predicted and result in unexpected effects
- the relative newness of gene editing technology, and whether there will be unanticipated long-term effects on human health or the environment
- how gene editing cannot be compared to conventional breeding because it can be imprecise and create genetic errors
2. Align with other regulatory systems
Generally, respondents who made comments about international alignment indicated that alignment with other systems is desirable. However, views on the preferred approach varied. Comments included:
- the CFIA should exempt plants developed using conventional breeding and edited plants that could have been developed using conventional breeding
- the CFIA should ensure Canadian growers have access to seed innovations, without unnecessary delay compared to the United States and other countries
- the CFIA should align more so with the European Union, and require mandatory review of plants derived from gene editing technologies
- Canada's product-based approach means that the CFIA is alone in capturing plants developed using conventional breeding, and from the experiences of other jurisdictions there is no indication that the CFIA's approach has prevented any unmanageable environmental impacts
- the CFIA should ensure internationally consistent import and export requirements
3. Clear guidance
Respondents indicated a need for clear guidance definitions and terminology.
Clear language and definitions
Many respondents said that the draft guidance lacked clarity in several definitions, processes, and information requirements. Respondents indicated that:
- the CFIA should provide plain-language seminars on the Seeds Regulations
- the CFIA should provide additional clarity around the pre-1996 exemptions for plants already in cultivation, including clearly identifying that historical cultivation and heritage cultivation by Indigenous communities qualifies for exemption from Part V
- definitions such as 'environment', 'conventional breeding', 'foreign DNA', 'gene editing versus. genetically modified', and 'new crop' were not sufficiently clear in the draft guidance, more detail was requested for stakeholders to better understand the scope of the terms
4. Impact on Canadian farmers
Gene editing technology was widely recognized as being a significant shift for agriculture. Some respondents expressed that this technology will bring opportunity for farmers and support sustainable agriculture and benefits for consumers. Other respondents expressed the desire for the CFIA to ensure Canadian plant populations remain free of gene-edited seed to support a wide variety of production methods for Canadian farmers, including organic production and market access where edited plant products would not be accepted.
Unintentional release into seed populations and natural areasFootnote 2
Respondents highlighted that edited plants could lead to unintentional release and impact farmers through:
- gene flow to weedy relatives or outcrossing to crops on neighbouring farmland, particularly for crop kinds where seed-saving is practiced or where gene flow can occur over long-distances, making co-existence challenging
- physical escape from farmland into the environment, particularly for weedy crop kinds that can naturalize, such as canola
Impacts on farmers
Many respondents commented on the challenges of maintaining the integrity of organic production. They identified that gene-edited seeds could complicate this situation through:
- uncertainty as to whether seed is free from gene-edited material
- reduced choice in seed purchasing decisions, including fewer crop rotation options if seed producers no longer offer suitable seed for some crop species
- increased costs associated with demonstrating that the crop meets organic standards
- loss of market access and reputational risks for the Canadian organic sector as a whole, should Canada be viewed as an unreliable source of organic products
- export complications, including the potential for export rejection by international organic markets
- difficulty meeting requirements outlined in the Canadian Organic Standards
Several respondents commented that the draft guidance did not address issues concerning competitiveness and access to innovative products, such as:
- the impact of over-regulation of conventionally bred plants on Canadian innovation
- the constricting of innovation in Canada due to unclear regulations
- the ability for Canadian farmers to access innovative plants quickly and efficiently
- disproportionate regulatory requirements for plant lines developed in Canada compared to imported plants
Other feedback
Feedback on topics that fall outside the scope of Part V of the Seeds Regulations was forwarded to the responsible office at the CFIA and other government departments including Health Canada, Environment and Climate Change Canada, and Agriculture and Agri-Food Canada. Examples included comments about:
- Canadian disadvantage for innovation and producer choice, including economic impacts related to
- decreased research investment due to regulatory burden
- limited access to seed that is suitable for organic production
- market loss due to unwanted presence of gene-edited seed
- trade disruption, including export rejection
- the Canadian Organic Standards
- labelling of gene-edited and genetically modified plants
- intellectual property rights and patent ownership of seed
- the safe and sustainable use of herbicides and pesticides
- issues related to food and food labelling
Next steps
The CFIA thanks everyone who participated in the consultation process. The CFIA has used input from this consultation to develop guidance that clearly communicates regulatory requirements and decisions, while supporting Canadian innovation and access to new technologies.