Table of Contents
- 1 About the Planning Guide
-
2 Recommended best practices
- 2.1 Key area of concern 1: Sourcing and introducing animals
- 2.2 Key area of concern 2: Animal health
- 2.3 Key area of concern 3: Facility management and access controls
- 2.4 Key area of concern 4: Movement of people, vehicles and equipment
- 2.5 Key area of concern 5: Monitoring and record keeping
- 2.6 Key area of concern 6: Communications and training
- 3 Glossary of Terms
- 4 Acknowledgements
- Appendix A: Writing a standard operating procedure (SOP)
- Appendix B: Sample visitors' log book page
- Appendix C: Bibliography
1 About the Planning Guide
1.1 Who should use this Guide?
This Planning Guide (the Guide) has been developed as an information resource for the National Farm-Level Biosecurity Standard for the Goat Industry (the Standard) to help goat producers prepare biosecurity plans for their farms. It is designed to serve the needs of goat producers across Canada, including all production types and herd sizes.
Producers are encouraged to prepare farm-specific plans, using both the Standard and the Guide. Individual farm plans will ensure that biosecurity measures suit the farm's size, layout and management practices.
Although on-farm biosecurity planning is primarily a producer's responsibility, family members, farm workers, advisors and others involved with the goat farm operation, often play an integral role in the implementation of the biosecurity plan and therefore can also benefit from using the Guide.
1.2 Why is biosecurity important for the goat industry?
Biosecurity is a set of practices used to minimize the transmission of disease-causing organisms in animal populations, including their introduction, spread within the population, and release.
Biosecurity is proactive and focuses on routine, day-to-day on-farm activities to protect the health of the herd by limiting the transmission of infectious agents that can cause disease in a goat or goat herd. Infectious agents are generally invisible, and can be moved from place to place in organic matter and on a wide range of materials that are frequently present in farming environments. Therefore, biosecurity focuses on reducing the risk of disease, on the assumption that infectious agents could be present.
For goat producers, biosecurity practices aim to achieve three general goals:
- Bio-exclusion: reducing the introduction of infectious agents to goats on the farm;
- Bio-management: reducing the spread of infectious agents among goats within a farm; and
- Bio-containment: reducing the spread of infectious agents between goat farms or from goat farms to other animal populations.
Biosecurity is important in reducing the risk and spread of endemic, economically-significant, production-limiting diseases, as well as avoiding catastrophic or foreign animal diseases. Reducing the risk of these diseases contributes to improved animal health, which creates the opportunity for day-to-day production improvement, cost savings and increased profitability, and protects the interests of both the producers and the industry as a whole.
Biosecurity practices can minimize the risk of exposure to zoonotic disease for producers, their families and their workers and reduce food safety risks potentially inherent in certain activities undertaken on the farm.
1.3 How to use this Guide
The Guide's best practices and resource information correspond with the organizational context of the Standard and focus on six key areas of concern:
- Sourcing and introducing animals
- Animal health
- Facility management and access controls
- Movement of people, vehicles and equipment
- Monitoring and records keeping
- Communications and training
The Guide is designed to assist producers in achieving the Target Outcomes identified in the Standard. The Standard also includes a set of self-assessment checklists that are the first step that producers should take when preparing or updating biosecurity plans for their farms.
The Guide provides a step-by-step planning approach and a detailed set of biosecurity best practices that will build on the self-assessments and help producers achieve the industry's Target Outcomes. They are intended to be adapted for use in each farm's biosecurity plans. In order to illustrate some of these best practices and important concepts, a number of case studies have been provided.
Biosecurity measures are closely related to both animal health and animal welfare practices; coordination of practices and measures among these important management disciplines is therefore recommended at the farm level.
It is important to note that the Guide, while comprehensive, is not a complete listing of all practices that could be used to meet the Target Outcomes. Other best practices may exist that support on-farm biosecurity.
A Glossary of Terms is provided in Section 3 of the Guide, and terms that are defined in the Glossary are bolded the first time they appear in the Guide.
1.4 Steps for developing a biosecurity plan
Here is a four-step process that will help you determine the risks within your goat operation, and select biosecurity practices to mitigate them.
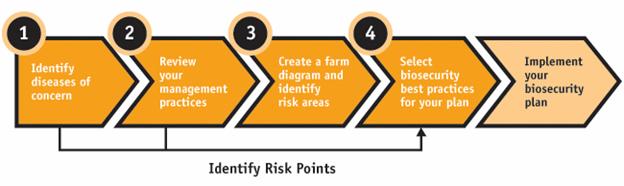
Description for Figure 1
The figure illustrates four steps in the development of a biosecurity plan and the final process of implementing the biosecurity plan. The steps are as follows: 1. Identify diseases of concern; 2. Review your management practices; 3. Create a farm diagram and identify risk areas; and 4. Select biosecurity best practices for your plan. In three of the steps (1, 2 and 4), points of elevated risk specific to your operation should be identified.
Each of the steps is described in some detail in the following sections. Here is an overview:
First, identify the diseases that are of greatest concern to you and your herd. Some of these diseases will be of concern industry-wide, some regionally, and some will be specific to your operation. Not all diseases you identify will necessarily be present in your herd, but you may wish to establish practices to keep them out. Each disease is transmitted in specific ways — for example, by direct contact with the infectious agent or as an aerosol, through manure, water and feed, or by contact with tools, equipment or any facilities contaminated with infectious material. Additionally, while some infectious agents are very fragile in the environment, some survive for days, weeks, months and even years. The modes of transmission should be understood for each of your diseases of concern.
The next step is to review your management practices, and note where infectious agents could be transmitted during your day-to-day activities. It is important to identify these risk activities and/or locations so that control measures can be effectively implemented. They may include the movement and commingling of goats and other animals, actions of people on the farm, use of tools and equipment, and contact between animals and facilities.
During this process, you should also prepare a diagram of your farm and identify where control zones and identified risk areas should be established. The controlled access zone (CAZ) and the restricted access zone (RAZ)are areas of your goat operation where access by people, vehicles and animals is managed, and specific biosecurity practices are implemented. Identified risk areas are areas on the farm within the CAZ or RAZ, such as isolation areas, areas of known infection risk (e.g., abscess or caseous lymphadenitis), kidding pens, and nurseries, where heightened biosecurity practices should be used in order to manage their inherently higher risk of disease transmission.
Step 1: Identify diseases of concern and disease-specific risks
The following questions can help you determine which diseases should be identified as diseases of concern on your farm:
- How common is the disease in goats in your region?
- What are the effects of the disease on your goats' health and productivity?
- How difficult is the disease to control?
- Is the disease zoonotic and/or reportable?
- Is it either present on your farm or a disease you wish to exclude from your farm?
Your herd veterinarian and goat disease experts can help you with this process. Table 1: Diseases of Concern is provided as a resource and lists a selection of diseases that may affect goats. The list is not a comprehensive list of all goat diseases, but will help you develop your list of diseases of concern.
Table 1: Diseases of Concern
Table 1: Diseases of Concern lists some diseases that may affect goats. The table identifies diseases that have the potential to be zoonotic, any other species that may be susceptible to the diseases, and the sources of infection. The last column in the table provides space for you to indicate whether the disease may be excluded from your biosecurity considerations or should be managed. If the disease is managed, there is also an opportunity to rate the management effort as low, moderate or high. At the bottom of the table there are several rows on which you can record any additional diseases of concern for your farm.
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Chlamydophila abortus (formerly Chlamydia psittaci) |
Y | Sheep, llamas, alpacas | Bacteria are shed in birth products, which can contaminate pasture and bedding. Bucks, via sexual transmission, and carrier does, including those infected as kids, are also sources of infection. Invades through mucous membranes (mouth, eyes, genital) and causes abortion at next pregnancy. |
|
| Q fever (Coxiella burnetii) |
Y | All animals including sheep, cattle, cats, dogs | Bacteria are shed in birth products, vaginal fluids, feces and milk and can be spread as an aerosol either from kidding does or contaminated dried bedding and manure. | |
| Toxoplasmosis (Toxoplasma gondii) | Y | Sheep | Oocysts are shed in the feces of cats (kittens) after eating infected mice. Cat feces contaminate feed (grain, forage) and pasture, which are then ingested by goats. Mice eat infected goat placenta and are a source of infection for the kittens. |
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Anthelmintic resistant (AR) gastrointestinal nematode (GIN) parasites | N | Sheep, llamas, alpacas | Failure to kill GIN parasites after deworming due to the parasites' resistance to that dewormer is an emerging problem. Inappropriate deworming practices can cause this resistance and goats are of particular risk because they a) fail to develop immunity to GIN parasites as adults and b) usually require a higher dose of dewormer to kill the parasites, so they are frequently inappropriately treated. New introductions pose a risk of bringing AR parasites onto a farm. |
|
| Coccidiosis (Eimeria spp) |
N | None | Oocysts are shed in the feces of infected kids and recovered adults and can build up in the environment (barn, drylot, pasture) until the load is high enough to cause disease in kids three weeks to six months of age. Fecal contamination of feed is associated with more severe levels of disease. | |
| Gastrointestinal nematode (GIN) parasites (Haemonchus, Teladorsagia, Trichostrongylus, Nematodirus) | N | Sheep, llamas, alpacas | Eggs are passed in feces of infected animals and contaminate grazing pastures. Introduced animals pose a risk of bringing in new infections. Adult goats can be affected as well. |
|
| Neonatal diarrhea (caused by coronavirus, rotavirus, enteropathogenic E. coli) | N | Lambs, calves, crias | Bacteria are shed in the manure of goats and can build up in the environment until the load in the kid rearing area is high enough to cause significant disease in kids less than two weeks of age. | |
| Neonatal diarrhea (caused by Cryptosporidia) | Y | Lambs, calves, crias | The oocysts of this protozoan parasite are shed in manure and contaminate the kidding and kid-rearing environment. If the load is sufficient, it can cause disease in kids two days to six weeks of age. | |
| Neonatal septicemia from opportunistic bacteria | N | Any very young animal | Kids that are born into a dirty environment and/or are colostrum deprived can contract bacteria from their environment. These bacteria enter through the navel or tonsils and invade the entire body. | |
| Orf / soremouth / contagious ecthyma (parapox virus) | Y | Sheep, llamas, alpacas | The virus lives in scabs, which drop off and contaminate the pens, feeders and hair. The virus can live for months to years in a dry environment. Some animals remain chronically infected and the virus can be isolated from scars of previous infections, and serve as sources of infection for other goats. | |
| Pneumonia (Mannheimia haemolytica, Mycoplasma ovipneumonia) | N | Sheep | These bacteria normally inhabit the throat of healthy goats. Environmental stressors (e.g., crowding, ammonia, temperature fluctuations, humidity, mixing of groups) will allow severe disease to occur. Occasionally respiratory syncytial virus (RSV) will cause acute, severe viral pneumonia in kids. The virus is shed in respiratory secretions from older recovered goats. |
|
| Pulpy kidney / enterotoxemia (Clostridium perfringenstype D) | N | Sheep | The bacterial spores are shed in feces and contaminate the ground and feed. If the animal lacks immunity and the feed source is rich (i.e. lush pasture, heavy grain), the ingested spores will grow in the gut, producing a toxin which rapidly kills the kid (sudden death). Adult does may also develop disease, but it is less acute. |
|
| Salmonellosis | Y | All animals | Feces from animals such as rodents, birds or other carrier animals contaminate feed. Diarrhea from infected animals contaminates the environment. |
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Caprine arthritis encephalitis (CAE) | N | Sheep | The virus is shed in respiratory secretions, which can be aerosolized, as well as in colostrum and milk. The virus infects goats of any age through the mucous membranes (respiratory tract, digestive tract, and conjunctiva). Sexual transmission and transmission in utero are also possible. Also causes hard udder, respiratory disease and arthritis. |
|
| Johne's disease | N | Sheep, cattle, deer, llamas, alpacas | Bacteria are shed in feces, colostrum and milk and are ingested by kids. The bacteria can survive for months to years in the environment. Transmission in utero from dam to kid also occurs. Shedding animals may not have symptoms of disease for several years. |
|
| crapie | N | Sheep | Reportable disease. Infected does will shed the prion in birth fluids and placenta at kidding. Prions contaminate the kidding grounds and infect other susceptible kids, adult goats and sheep, if present. Milk and urine are also sources of infection. |
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Foot scald (Fusobacterium necrophorum) |
N | Sheep | The bacteria are ubiquitous in the environment. They can invade the soft tissues between the toes if the environment is dirty and wet, or if there is a wound or other foot trauma. |
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Deer meningeal worm (Parelaphostrongylus tenuis) | N | Sheep, llamas, alpacas | This parasite, whose host is the deer and cycles through land snails and slugs, can infect goats if the goat inadvertently eats the infected snails and slugs. The parasite invades the central nervous system, causing disease. | |
| Listeriosis (Listeria monocytogenes) |
Y | Sheep, cattle | The bacteria are present in the soil and digestive tracts of many mammals, including rodents. Goats become infected after ingesting silage and other feeds that have been contaminated with feces. Growth of the bacteria is fostered by cool, wet conditions at normal to high pH. Also causes abortion and pink eye. |
|
| Rabies | Y | All warm-blooded mammals | Reportable disease. Contact with wildlife, most commonly foxes and skunks, is the main source of infection. Unvaccinated farm cats and dogs also pose a particular risk because of close contact with livestock and humans. | |
| Tetanus / lockjaw (Clostridium tetani) |
Y | All animals | The bacterial spores can live for decades in the soil. If the animal has a wound or kidding injury, it can become contaminated with the spore-containing soil. The bacteria then grow in the wound and produce a toxin which is absorbed by the nerves and causes disease. |
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Biting and sucking lice | N | None | Nits (eggs) and lice are transmitted by direct contact between animals, contaminated tools, equipment and bedding. | |
| Caseous lymphadenitis (CLA) (Corynebacterium pseudotuberculosis) | N / Y | Sheep, llamas, alpacas | The bacteria come from broken abscesses and from the lungs when abscess material is coughed up. They contaminate pasture and feed and can survive for days (water) to weeks (feed) to months (soil, feeders, grooming equipment). The bacteria then invade through skin and cuts in the mouth. CLA is an important cause of chronic wasting. |
|
| Dermatophilosis (D. congolensis) |
Y / N | All mammals | The bacterial spores commonly occur in the environment. Disease usually occurs when the animal is housed in dirty and wet conditions. | |
| Flystrike | N | All animals | The green-bottle fly (Lucilia sericata) is attracted to decaying organic material and will lay its eggs on live animals that are wet or dirty. Animals with diarrhea, wounds, and foot rot, are very susceptible to maggot infestation, which causes illness and death. Poor management of deadstock may attract more flies. | |
| Pink eye (Mycoplasma conjunctivae & Chlamydophila pecorum) |
N | Sheep, not cattle | Goats can be carriers of the bacteria and shed the bacteria in lacrimal secretions. Outbreaks occur when groups are mixed or new animals are introduced. | |
| Ringworm (fungal infection) |
Y | Sheep, cattle | The fungus prefers dark, moist conditions and can survive for a prolonged time in the environment. Transmission occurs easily through direct contact, as well as via grooming tools and equipment and shared pens at shows. |
| Disease Category and Name |
Zoonotic (Yes=Y/ No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
| Mycoplasma mycoides subsp. mycoides | N | None | The bacteria can be spread through contaminated milking equipment and hands, and is highly contagious. Does may shed the bacteria in seemingly normal milk. Kids drinking infected milk will develop septicaemia and joint ill. | |
| Staphylococcus mastitis | Y | All animals | The bacteria are commonly present in skin infections (including people) and can be transmitted in many ways including milking, nursing kids, teat wounds, soremouth/orf lesions on the teats, dirty hands, poor udder preparation for milk, and lack of teat dipping. |
| Disease Category and Name |
Zoonotic (Yes=Y/No=N) |
Other Susceptible Species | Sources of Infection | Your need to exclude or manage (Low=L, Moderate=M, High=H) |
|---|---|---|---|---|
There are a number of reportable diseases of goat, including some mentioned in this table (e.g., rabies and scrapie). For information on federally reportable diseases, visit the Canadian Food Inspection Agency's website. Some provinces also have a list of reportable diseases. Contact your respective provincial organization for more information (see section 1.5).
Identifying your diseases of concern will help you identify the risks on your farm. It is particularly important to know how they can be transmitted to your goats. As illustrated in Table 1, some diseases are transmitted directly from one animal to another, for example:
- direct contact (e.g., nose-to-nose contact, venereal transmission during breeding) or exchange of secretions or excretions (e.g., saliva);
- ingestion of infected milk or colostrum; or
- vertical transmission from dam to the fetus in utero.
Many diseases are also indirectly transmitted, for example:
- ingestion of feed, pasture, or water that has been contaminated with manure, urine, birth fluids or placenta. It is important to note that contamination may be from an infected or carrier animal, or from contact with pens, pathways, pastures, or vehicles that were previously contaminated;
- suckling from dirty teats contaminated with manure;
- ingestion of or contact with feed, water, pasture or bedding that has been contaminated by cats, dogs, rodents or other wildlife, which may be carrying an infectious agent; or
- contact with hands, boots, tools and equipment that are contaminated with infectious agents (e.g., contaminated hands during the milking process).
This information will help you identify the risks of disease transmission during your day-to-day and seasonal activities as you operate your farm.
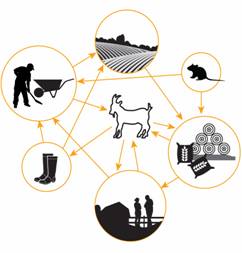
Description for Figure 2
The conceptualized diagram illustrates that infectious agents may be transmitted in a number of ways. These transmission pathways include transmission from goats to boots to equipment, pastures, people, bedding and feed; from equipment to goats, pastures, people, bedding and feed; from people, bedding and feed to people and goats; and from rodents to equipment, bedding and feed.
Not all biosecurity practices are disease-specific. Many diseases are transmitted in similar and common ways; therefore, some proactive biosecurity practices will reduce the risk of transmission of multiple diseases.
Step 2: Review your management practices
You may have a set of management practices or standard operating procedures(SOPs) for your farm. If you do not have a set of SOPs, begin by preparing a simple outline of the activities that you and your farm workers carry out on a regular basis.
First, list the work activities you perform on a daily, and weekly basis, and those that you perform over a longer period, monthly, seasonally, and annually. Create a list of the steps that are carried out when completing each activity. Include a note about who performs each activity, what equipment is used, and where the activity usually takes place, including when the animals are moved from place to place.
Now think about the steps in each activity and make a note beside any that pose a risk for the spread of infectious agents, if present. Consideration should be given to areas or activities where goats and other animals, people, equipment, inputs (e.g., feed, bedding, sample collection containers), vehicles and/or your facilities have the potential to come in contact with an infectious agent. It is at the potential point of contact and/or point of introduction to your farm that biosecurity measures should be implemented to minimize the risk of disease introduction and spread.
Step 3: Create a farm diagram and identify risk areas
The concept of controlled access zones (CAZs) and restricted access zones (RAZs) has been accepted internationally and adopted by some livestock sectors in Canada. This approach is used to identify relatively large areas of a farm for biosecurity management and is the first line of defence on your farm.
Purpose of zoning
The purpose of zoning is to isolate the herd from infectious agents introduced by infected or contaminated animals, people, tools, equipment, vehicles, feed, water and pests entering the zone, and to contain any issues within the herd.
The controlled access zone (CAZ) outlines boundaries that encompass all of the active production areas of the farm, including the restricted access zone (RAZ) and the areas where service activities involving people, equipment and supplies are conducted. The CAZ boundary may be marked with signage and have a physical boundary. Certain biosecurity practices are in place for animals, people, equipment, inputs and vehicles prior to entry into the CAZ, which preferably occurs through a pre-established controlled access point (CAP).
The RAZ is a defined and identified zone within the CAZ and includes the areas where the herd is maintained. Therefore, the RAZ is intended to limit unnecessary entry, allowing access only under pre-defined biosecurity conditions. Specific biosecurity practices are in place for animals, people, tools, equipment and vehicles prior to entry into the RAZ. Access to the RAZ should be through a controlled access point.
Designating and managing additional identified risk areas within the CAZ and the RAZ will reduce the risk of the spread of disease between different animal groups within the herd or between an individual goat and the herd by minimizing exposure of animals of differing health status or susceptibility. Dedicated tools and equipment for use in each area or implementation of biosecurity protocols for cleaning and disinfection are important components in managing identified risk areas.
How zones work
- They are risk-defined: A risk assessment of the production activities is undertaken and their specific disease risks are determined. In order to effectively designate zones, appropriate biosecurity practices must be implemented in these areas so that specific risks can be managed.
- They are secure: They are often physically defined by walls, fences, secure doors and gates to ensure that animals and people cannot freely move into or out of a zone.
- They are visible: Biosecurity zones are clearly identified and people are notified of the zone-specific practices for entering, exiting and circulating with the zone.
- Access by people is managed: Access by and movement of people (i.e., farm workers, family members, service providers and visitors) are deliberately managed to support bio-exclusion, bio-management and bio-containment.
- Animal movement is managed: Farm workers are aware of the risks of disease transmission associated with animal movement into and throughout the premises. Movement is planned to mitigate these risks.
- Transition points are identified: There is a visually-defined entry point through which all traffic (vehicles, people, animals, inputs and equipment) will enter a CAZ and a RAZ, often referred to as a controlled access points (CAPs). Specific biosecurity protocols may be in place at access points; for example, tools and equipment may be limited to use in only that area or specific cleaning and disinfection may be required. Hands may be washed and protective clothing may be changed (e.g., coveralls) or cleaned (e.g., footwear).
- They are specific to each operation: The size and complexity of each operation and its existing facility layout will contribute to zoning decisions; i.e., a small, integrated meat herd that is essentially housed and handled together will employ a different zoning strategy than a larger dairy operation that operates as a closed herd, or an integrated breeding and meat operation.
Preparing a farm map and zones
The following diagrams are examples only and are not intended to be directly applied to your specific farm, production type and operational management. The examples do include some common or general farm layouts to assist you in determining the most appropriate designation of biosecurity zones and/or identified risk areas specific to your operation.
Sketch your farm layout
Using a pad and pencil (or working on a printed aerial photo, Google® or other map of your farm) prepare a simple map or diagram of your farm.
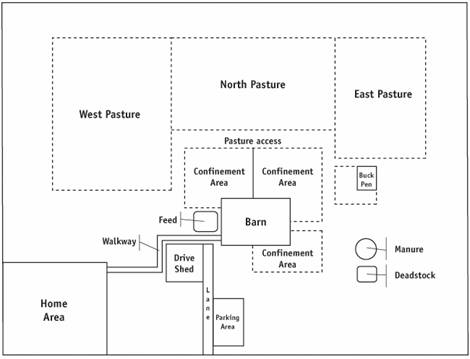
Description for Figure 3
The figure illustrates a conceptual example of a farm layout, including:
- Home area
- Farm buildings:
- Barns
- Drive shed
- Confinement areas
- Animal loading area
- Feed storage area
- Manure storage area
- Deadstock pickup area or compost location
- Barn office
- Driveways and lanes
- Parking areas
- Paths and walkways
- Pastures
- Pastures and housing areas for other animals on the farm
Select the restricted access zone (RAZ)
Identify the production areas in which goats should be protected from exposure to infectious agents from outside the farm and in which they should be protected from infectious agents within the farm. Also consider areas of potential traffic that are essential to the production area and the associated risks of disease transmission. There are various options, depending on farm layout and production practices.
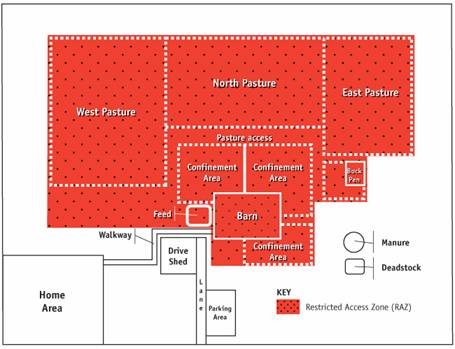
Description for Figure 4
This figures illustrates how on many farms, the restricted access zone (RAZ) includes all of the production areas and the pasture areas. This option creates a single zone for the full production facility and therefore reduces the number of times that goats, farm workers and others will move from zone to zone and carry out the required procedures. The RAZ includes the west, north and east pastures, the pasture access, the three confinement areas, the feed storage, the barn and the buck pen.
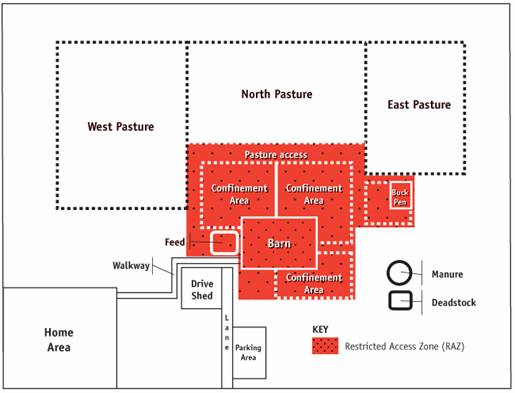
Description for Figure 5
This figure illustrates the option of having the RAZ include all of the working areas of the farm, but exclude pastures. This assists farms where defining a security zone that includes pastures is impractical. The RAZ includes the three confinement areas, the feed storage, the barn and the buck pen. This approach applies to farms with larger pastures and/or pasture areas that are not well-defined, or difficult to control. It allows a concentrated effort on biosecurity in the areas frequently used by the herd, farm workers and service providers and, therefore, the highest risk areas.
Select the controlled access zone (CAZ)
Once the RAZ has been determined, consider the areas that should be designated as the controlled access zone (CAZ). The CAZ contains operational facilities indirectly involved in animal production. It includes the areas in which service providers and farm workers would circulate before entering and after leaving the production area, and where they conduct their activities when they are not actively engaged with the goats (e.g., laneways, parking areas and equipment sheds). The CAZ encloses the RAZ.
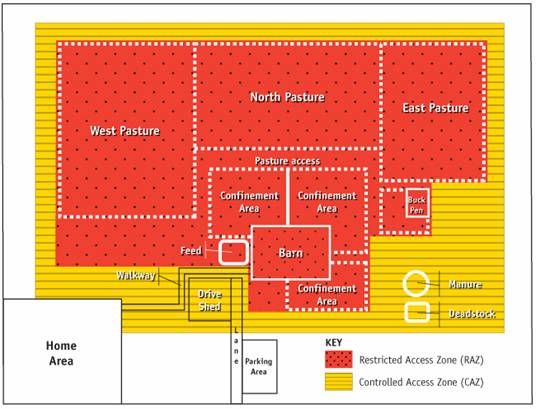
Description for Figure 6
In this image, the RAZ includes pasture, and the CAZ surrounds the RAZ and serves as a buffer for all of the areas where goats may be found. It also includes the barn office, walkway, drive shed, a portion of the laneway, and manure and deadstock storage. With this approach, there is a relatively small border around the entire farm that provides a transition to and from areas of the farm not directly used in goat production.
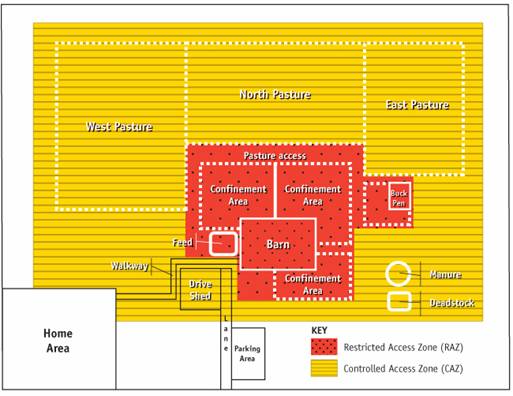
Description for Figure 7
In this image, the RAZ is designed to include only the more frequently-active goat production areas (e.g., barn, confinement area). The pastures and surrounding area (e.g., walkways) will be included only as part of the CAZ. This zone will be used to define the required practices and will also contain, as in Figure 6, the indirect production activities and enclose the RAZ. The controlled access zone (CAZ) includes the west, north and east pasture, the barn office, the walkway, drive shed, part of the laneway, and the manure and deadstock areas.
Identify controlled access points (CAPs)
When the zones have been applied to the farm's physical layout and its production practices, access points should then be identified. Controlled access points (CAPs) are a limited number of places at which people, animals, equipment, vehicles and inputs may enter and exit the zones. Some of these will be located where secure gates and doors, lanes and pathways already exist. Others will be defined by specific activities; for example, the movement of manure, the location of sick pens, and the delivery of feed.
An anteroom is recommended as the main access for people entering or leaving the RAZ. The anteroom is a transition area, which may also be the controlled access point, and facilitates the implementation of biosecurity protocols. In the anteroom, there should be a visual or physical marker which acts as the point for the implementation of biosecurity protocols. This may be a line painted on the floor, or a wall, wide bench or other type of barrier. For example, biosecurity protocols such as change of boots, outer clothing, hand washing, equipment cleaning and disinfection, should occur prior to crossing that point.
Controlled access points (CAPs) are usually physically identified, and specific practices should be followed whenever animals, people, equipment, inputs and vehicles move into or out of the zone. These are the critical points at which biosecurity practices can be implemented to minimize the risk of introducing and spreading infectious agents.
Determine identified risk areas
Copy the main production areas from your farm map/diagram onto another sheet in larger scale. It will be useful to keep them in similar relative positions and in somewhat similar proportion as in the farm map. Identify the activities that are undertaken in each of the areas on this diagram, both outside and inside the barn or the main production structure.
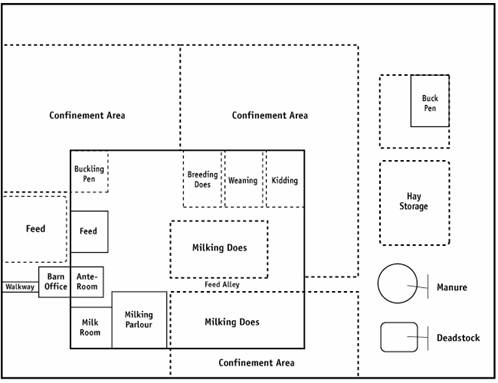
Description for Figure 8
The figure shows the main production area layout, which may include milking doe pens, buck pens, buckling pens, kid-rearing pens, weaning pens, breeding doe pens, kidding pens, isolation areas, hospital or sick area, breeding areas, handling areas, milking parlour and house, confinement areas, feed storage, manure storage and deadstock disposal.
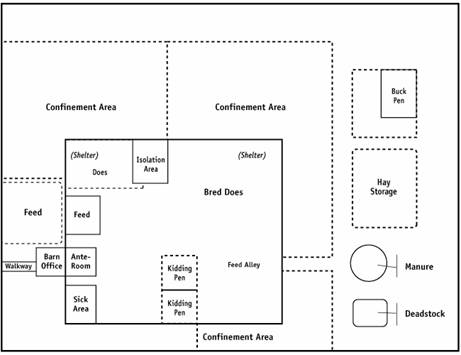
Description for Figure 9
This image shows the main production areas on a meat goat operation, which may include the buck pens, breeding doe pen, dry doe pen, kidding pens, isolation area, hospital or sick area, confinement areas, feed storage, manure storage and deadstock disposal.
Looking at these and other areas that are designated on your farm for specific activities, conduct a risk assessment of the areas. This may be as simple as classifying areas as low, moderate or high risk of disease transmission, based on:
- the health status and susceptibility of the goats that could be brought into the area;
- the nature of the activity;
- the goat's length of stay in the area;
- the likelihood of contact with other goats; and
- the anticipated traffic of farm workers, service providers and visitors.
It is also important to consider:
- areas where all visitors are allowed;
- areas where some or all visitors are restricted (i.e., should change clothing, wash and/or sanitize hands and footwear before being admitted); and
- areas where animals of differing health status are housed (e.g., new introductions, diseased animals or animals of unknown health status, sick animals undergoing treatment, animals on a health program).
Step 4: Select biosecurity practices for your plan
At this point in the preparation of your biosecurity plan, you have:
- determined your diseases of concern;
- reviewed your farm management activities; and
- designed your farm zones and identified risk areas.
It will be beneficial to also refer to the National Farm-Level Biosecurity Standard for the Goat Industry.
The Standard contains a self-assessment checklist for each key areas of concern highlighted in this Guide, as well as a worksheet to outline your biosecurity gaps and goals. These tools will help you focus your efforts on areas that require special attention on your farm.
Section 2 of the Guide provides a comprehensive collection of recommended best practices. These best practices are designed to reduce the risk of disease transmission:
- within your herd;
- within your facilities;
- in your day-to-day activities; and
- in the actions of workers, service providers and visitors on your farm.
After reviewing the biosecurity best practices, choose the practices that support the goals and address the gaps you have identified. Once you have worked through the sections of the Standard and the Guide that are relevant to your operation and put all of the required information together, you will have completed a biosecurity plan that is specific to your farm and will help you address your risks and promote a healthy goat herd.
1.5 Biosecurity resources
One the best sources of information for biosecurity and any other animal health-related topic is your herd veterinarian. Large animal veterinarians have extensive training and experience in the principles of biosecurity in livestock production that may be applied to goat production. To assist you in locating a veterinarian, the veterinary medical association for each province is provided below. Each association has a database of veterinarians and veterinary clinics for that province.
- College of Veterinarians of British Columbia
- Alberta Veterinary Medical Association
- Saskatchewan Veterinary Medical Association
- Manitoba Veterinary Medical Association
- College of Veterinarians of Ontario
- Ordre des médecins vétérinaires du Québec (French only)
- New Brunswick Veterinary Medical Association
- Nova Scotia Veterinary Medical Association
- Prince Edward Island Veterinary Medical Association
- Newfoundland and Labrador Veterinary Medical Association
For additional information on biosecurity, goat diseases and animal health regulations, the Canadian Food Inspection Agency of the Government of Canada is a valuable resource.
Many provincial governments have supplementary resources on goat herd management and biosecurity. There may also be provincial veterinary extension specialists available for your herd veterinarian to contact and gather additional information.
- British Columbia Ministry of Agriculture
- Alberta Ministry of Agriculture and Rural Development
- Saskatchewan Ministry of Agriculture
- Manitoba Agriculture, Food and Rural Initiatives
- Ontario Ministry of Agriculture, Food and Rural Affairs
- Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (French only)
- New Brunswick Department of Agriculture, Aquaculture and Fisheries
- Nova Scotia Department of Agriculture
- Prince Edward Island Department of Agriculture and Forestry
- Newfoundland and Labrador Department of Natural Resources
For current information on the goat industry in Canada, visit the Canadian National Goat Federation. There are also a number of provincial and sector-specific associations that may be applicable to your farm operation. The list below is not exhaustive, but highlights some of these associations.
- Canadian Goat Society
- Canadian Meat Goat Association
- Canadian Cashmere Producers Association
- British Columbia Goat Association
- Vancouver Island Goat Association
- Alberta Goat Breeders Association
- Manitoba Goat Association
- Saskatchewan Goat Breeders Association
- Ontario Goat
- Le Regroupement des Éleveurs de Chèvres de Boucherie du Québec (French only)
- Le Syndicat des Producteurs de Chèvres du Québec (French only)
- New Brunswick Goat Breeders Association
- Goat Association of Nova Scotia
Additional information on developing and implementing biosecurity plans may be found at the following sources:
2 Recommended best practices
In this section, there are a number of best practices for you to consider incorporating into your farm's biosecurity plan. The information provided aligns with section 2 of the Standard and will assist you in achieving the Target Outcomes.
2.1 Key area of concern 1: Sourcing and introducing animals
Target Outcome: Animal introductions and re-entry, and the use of semen and embryos do not present a risk to the health status of the herd.
2.1.1 Sources and sourcing
Producers can reduce the risk of introducing diseases onto their farms by controlling the way they source and introduce new animals and/or source artificial insemination and embryo transfer materials. Sourcing semen and embryos from accredited suppliers, limiting the frequency of animal additions, and controlling the number and quality of sources of new animals will reduce the risk.
- Raise as many replacement goats as possible on your farm, and only add new goats from off-farm sources when necessary.
Do you have a closed herd?
A closed herd is one which has no new animals enter and no animals leave (e.g., to shows) and then return. New genetics are introduced only through the use of artificial insemination or embryo transfer. Most goat herds are not closed but may occasionally source animals from outside the herd.- Ensure the reproductive efficiency of your current herd by optimizing fertility through breeding management programs for your does and breeding soundness examinations (BSEs) for your bucks. Adequate nutrition also plays an integral role.
- eproductive technologies to introduce new genetics, such as artificial insemination (AI) or embryo transfer (ET), rather than purchasing animals from outside sources.
- Know the biosecurity and animal health practices of all replacement animal suppliers. Ask the following questions:
- What is the health status of their herd? (see section 2.1.3)
- What biosecurity practices do
- Adopt protocols to improve the survival rate of kids in your herd. This includes colostrum management, appropriate treatment of hypothermia and hypoglycemia, control of important infectious diseases (see Table 1) and appropriate housing. For meat goats, mothering of kids is also important.
- Consider using reproductive technologies to introduce new genetics, such as artificial insemination (AI) or embryo transfer (ET), rather than purchasing animals from outside sources.
- Know the biosecurity and animal health practices of all replacement animal suppliers. Ask the following questions:
- What is the health status of their herd? (see section 2.1.3)
- What biosecurity practices do they employ?
- Do they commingle animals from different sources?
- How do they transport their goats?
- How does the health status of the source herd compare with that of my own herd?
Case Study: "Sourcing New Stock"
A producer wants new genetics in her herd, but lives in an isolated area. She finds a breeder (a 24 hour drive away) with a herd health status compatible with that of her own. The producer selects the young kids (two to three at a time) that she wishes to purchase from the breeder. She ships an empty cleaned and disinfected dog crate to the breeder. The breeder cleans and disinfects the crate, adds fresh bedding, puts the tagged kids into the crate and drives to the airport to ship the kids by air to the producer (a two and a half hour flight). The producer receives the kids on the other side, moves them into isolation, and cleans and disinfects the crate. The producer is buying young stock from a reputable breeder, avoiding commingling, and ensuring the safest and quickest mode of transport for the young animals. - Purchase goats from suppliers with known health status and health status that is compatible with (equal to or higher than) your herd.
- Encourage breeders and sources of goats and kids to adopt biosecurity practices throughout their operations, and to make their disease-management and health-status records available to prospective purchasers, including their participation in the national Scrapie Certification Program for goats. Visit Scrapie Canada for more information.
- Plan ahead for additions so arrangements can be made with the chosen, reputable suppliers.
2.1.2 Biosecurity practices at fairs, shows and off-site loan locations
When fairs, shows and other off-site locations provide inadequate biosecurity for visiting goats, there are proactive measures that producers may implement to reduce the risks. These include transporting their animals in home-farm vehicles; bringing feed, water, water-delivery systems and other needed equipment from the home farm; applying biosecurity protocols at the off-site location; and treating returning goats as new arrivals. Producers should inquire about whether the fair or show has a biosecurity policy and a set of procedures to minimize the risk of disease transmission. With no policy in place, evaluating risks and having the capacity to minimize risk become part of the decision-making process when considering whether to attend the off-site event.
- Know the biosecurity practices at any fairs, shows or off-site loan locations before attending.
- Conduct a risk assessment for each off-site activity in which you plan to participate based on the nature of the activity, the biosecurity practices in use at the site, and your ability to implement additional biosecurity practices as needed.
- Attend only those fairs, shows and other off-site activities that are suitable based on your risk assessment and/or that have biosecurity programs that are suitable for your goats. It is recommended to:
- Ensure that a mandatory veterinary inspection of all goats attending the show is conducted prior to unloading, and that any goat with evidence of an infectious disease is not unloaded at the show;
- Ensure that goats that have recently kidded (i.e., within the last two weeks) or may kid at the time of the show are excluded from the show grounds due to risk of transmission of infectious abortion diseases;
Case Study: "Biosecurity at Fall Fairs"
A group of goat producers who like to show their goats have formed a biosecurity group and developed a set of recommendations for the fall fairs in their provinces, which host those shows. The document suggests that the fairs' committees institute the following recommendations: separated penning for each exhibitor's animals to prevent direct contact between goats of differing health status; minimal time at the show to reduce risk; veterinary inspection of all goats for evidence of infectious disease prior to unloading at the show; mandatory hand sanitization between handling of the goats (e.g., by the judge). Signage should also be posted, requesting that the public does not touch the goats, and hand-washing stations should be available for use by the public and exhibitors. As a result of the producers' lobbying, the fall fairs adopted these measures, thus reducing risk to the animals. - Require that the fair board ensure the proper cleaning and disinfection of the available facilities prior to the goats' arrival; and
- Ask that signage be posted to indicate to the public that goats should not be handled in order to protect the health of the goats.
- Limit the time that your goats are at the off-site location.
- Transport your goats in a vehicle that has been cleaned and disinfected prior to use. Ideally, this vehicle is dedicated exclusively to your farm's use.
- Prevent commingling and direct contact and limit proximity with other goats and livestock in transit and on-site.
- Supply bedding and feed from your home farm.
- Ensure a clean supply of water on-site.
- Bring feeders, water buckets, and grooming and handling equipment from your home farm for exclusive use on your goats.
- Limit handling of your goats by others, but when it is necessary (e.g., for the judge's examination) require that handlers wash and/or sanitize their hands before contact with the animals and before handling the next animal.
2.1.3 Disease status at purchase or re-entry
Knowing the health status of new animals and animals that are re-entering the farm (e.g., animals attending livestock shows, borrowed or loaned goats) allows producers to implement biosecurity measures to minimize the risk of introducing and spreading disease to their existing herd.
- Obtain the following information from the vendor prior to purchase:
- the animal's health records, including diseases and treatment, the type and timing of vaccinations and other preventive health measures (e.g., deworming)
- the vendor's testing protocols for your diseases of concern
- the vendor's diagnoses of your diseases of concern
- the health management plan of the source herd
- Consider testing new or returning animals prior to introduction or re-introduction, in consultation with your herd veterinarian, when appropriate and specific to the diseases of concern for your herd. Tests used to determine disease status can include serology, culture, and fecal egg counts. Testing may also include a clinical examination by a veterinarian. In some cases, disease testing can be done prior to purchase but, in any case, the animal should remain in isolation until the test results are known. Have a plan for animals with positive test results; e.g., treat, do not buy.
Testing for Disease
Diagnostic testing for disease is very complicated and its accuracy depends on which disease is being tested for and on the goat's stage of infection. An accurate test means that an infected animal will always test positive, and an uninfected animal will always test negative. For some diseases of concern, there are no individual animal tests with sufficient accuracy that will entirely remove the risk of misclassifying a goat's disease status (i.e., deem an infected goat healthy) when purchasing a replacement animal. For this reason, the introduction of new or returning animals will always carry some risk of disease transmission. Please speak with your herd veterinarian about testing for the diseases of concern in your herd. - Obtain all identification records and herd of origin information (if different from source herd).
- Follow protocols when purchasing semen or embryos. It is recommended to:
- Purchase semen from a donor buck collected in a CFIA-accredited semen production centre;
- Purchase semen from international sources only if it meets Canadian regulations; and
- Purchase and transfer embryos only from source animals with known and low-risk disease status.
2.1.4 Isolation upon arrival or re-entry
Isolating new or re-introduced animals for a sufficient time helps to identify animals with acute infections that are still in their incubation period, allowing for the shedding of infectious agents to cease, and to carry out testing and treatment. Some infectious agents may be difficult to detect within a normal isolation period.
- Designate an isolation area that is separated from other goats and livestock on the farm, with no opportunity for direct contact. The isolation area should be separated from the main herd by a solid partition and a secure door, or be located in a different building. The airspace should be separately ventilated to prevent transmission of airborne diseases, including caprine arthritis encephalitis virus and Coxiella burnetii (Q fever).
- Isolate all newly-acquired animals prior to introduction to your home herd for a period of time suitable for your diseases of concern, and:
- monitor them for disease;
- administer any required vaccines; and
- implement an isolation protocol in consultation with your veterinarian for elimination of anthelmintic-resistant gastrointestinal nematode parasites.
- Ensure that there is no contact between people or equipment and the isolated animals without sanitation practices, including hand washing or use of dedicated disposable gloves, a full change of outerwear and cleaning and disinfection of any equipment, tools and footwear. This applies both upon entry into and exit from the isolation area.
Using Gloves
Gloves are only effective at limiting the transmission of disease if they are used correctly. When gloves are dirty, they are essentially the same as dirty hands. Gloves should be used only when handling one goat or group of goats within the same pen and be disposed of after each use. Hands should also be washed before and after using gloves. - Prevent any sharing of feeding or watering equipment, penning, handling facilities or equipment— including milking equipment— between isolated goats and resident goats unless they are first cleaned and disinfected. This includes shared walkways (e.g., to the parlour and back).
- Clean and disinfect the isolation area regularly, including after each use. Design the area to facilitate cleaning and disinfection practices.
2.1.5 Protocols for releasing animals from isolation
Isolation will only be effective if there are protocols in place for releasing animals at the appropriate time.
- Observe no clinical signs of disease during the isolation period.
- Quickly attend to all animals showing signs of clinical disease. Test, treat and/or cull.
- Complete all disease testing, treatments, procedures and vaccinations before goats are released from isolation.
2.2 Key area of concern 2: Animal health
Target Outcome: Animal health, well-being, and productivity will be optimized through proper implementation of herd health programs.
2.2.1 Implement a herd health management program
A herd health management program identifies the key components required for appropriate disease prevention, control, and treatment for each farm. The herd veterinarian is a key partner for determining the specific risks to the herd and then designing and implementing the herd health management program.
Health management refers to those procedures that are followed to prevent and control disease and optimize health and performance. Most goat producers have a herd health management program that they follow to maintain the health of their goats, including guidance on vaccinations and other preventive measures and disease identification and management. Often, these programs have been prepared with the assistance of a veterinarian and/or input from other specialists and advisors.
Table 2: Components of a Herd Health Management Program
Table 2 provides reference information to evaluate your herd health management program for completeness. The recommended components of the program are highlighted with some key details.
| Component | Details |
|---|---|
| Herd health visits | Schedule visits and communicate with the herd veterinarian regularly Review herd health programs frequently, depending on the health status of the herd |
| Monitoring health | Record disease and health events Analyze records to detect health issues that require action Test for disease (e.g., fecal egg counts, necropsy of kid mortalities) |
| Nutritional health management | Complete a routine assessment of body condition to detect nutritional issues that will affect health and productivity Provide adequate vitamin and mineral nutrition with specific attention to iodine, selenium and vitamin E Conduct feed analysis and water testing |
| Reproduction program | Establish a breeding schedule Complete ultrasound examinations for pregnancy Plan lighting and/or hormonal strategies for out-of-season breeding Conduct a buck breeding soundness examination |
| Udder health | Perform udder preparation and teat dipping Monitor for evidence of mastitis Establish a milking order Ensure adequate management of dry does |
| The periparturient period | Disinfect navels with a suitable product Heat treat colostrum to 56 degrees Celsius for 1 hour prior to administration Ensure appropriate colostrum intake; If hand feeding, the first feeding should ideally occur within the first hour (50 ml/kg of body weight 4 times (every 6 hours) in first 24 hours of life) Pasteurize milk for kids Attend to dystocias and any evidence of illness in the periparturient period such as pregnancy toxaemia and milk fever |
| Kid rearing | Investigate kid mortality Be aware of the diseases of concern for kids, including coccidiosis, pneumonia, hypothermia and hypoglycemia, neonatal diarrhea, pulpy kidney, and orf/soremouth |
| Disbudding and castration | Perform with suitable timing and technique and with concern for animal welfare Consider vaccinating for clostridial diseases, especially tetanus, before the procedures |
| Vaccination programs | Establish an appropriate regime for each age and/or production group of animals that is specific to your farm and diseases of concern; include clostridial diseases (e.g., tetanus, pulpy kidney / enterotoxaemia – primary series and annual vaccinations) |
| Parasite control program | Set up a gastrointestinal parasite control program that manages pasture contamination, uses anthelmintics appropriately, and monitors animals for internal parasites Treat animals selectively Isolate and treat new introductions Investigate treatment failure Control external parasites |
| Disease control programs | Implement specific disease control programs, if required, to control and eradicate an infectious disease; examples are CAE, infectious abortion, CLA and gastrointestinal parasites Use tools such as disease testing, including necropsy of dead or dying animals, biosecurity, vaccinations and management changes |
| Treatment protocols for sick goats | Apply an isolation procedure for sick animals Follow SOPs written in consultation with the herd veterinarian to manage simple disease conditions Prepare meat and milk withdrawal times in consultation with herd veterinarian and CgFARAD, since there are no licensed veterinary drugs for goats in Canada |
| Euthanasia | Use appropriate handling and restraint, as per the standards of the World Organisation for Animal Health and welfare Codes of Practice Complete with a method that complies with CFIA humane destruction recommendations |
| Federal and provincial disease monitoring programs | Participate in the Voluntary National Scrapie Program, which at the time of preparation of the Standard and the Guide, was the only program of its type |
| Disease / emergency response plan | Employ enhanced biosecurity practices when a disease outbreak is suspected or confirmed on your farm or in your area Contact a federal veterinarian in the event of a suspected reportable disease |
Your herd health management plan will provide important information as you develop and implement a biosecurity plan on your farm. The plans complement each other to proactively reduce the risk of diseases entering and spreading within your farm. Effective biosecurity implementation may also support your disease response plan.
If you have other livestock on your farm, consider developing herd health management programs for the other species as well, with particular attention to addressing disease and health risks that are shared among the species on your farm.
2.2.2 Observe and evaluate the health of animals
Regular observation of the goats for any change in health status is conducted on a daily basis, and the findings are recorded. Trigger points exist to help direct further action and to allow a rapid response to potential disease.
- Walk through your herd at least once each day. Attention should be paid to:
- attitude and behaviour
- gait
- body condition and rumen fill
- interaction with other herd mates, including dam with kid(s)
- any clinical signs of disease; e.g., diarrhea, depression, abortion, lameness, abnormal discharges
- Record observations from these walk-throughs, including:
- significant deviations from normal behaviour in individual goats and in the herd, and
- changes in appetite and/or milk production of individual goats
- Establish a set of trigger points. Trigger points are observations which, when noted, lead to further action (e.g., percentage of drop in milk production, cluster of abortions, blister around an animal's mouth or hoof).
- Move any animal that is exhibiting clinical signs of disease or is of unknown health status to an isolation area. These animals should be isolated separately from any new additions or returning animals. See section 2.1.4 for more information on designing an isolation area.
- Contact your herd veterinarian for assistance with further examination, diagnostic testing and treatment, if required.
- Have a hospital pen for sick animals. This area separates sick animals from the rest of the herd, and facilitates treatment and ongoing monitoring. The principles of designing and maintaining a hospital pen are similar to those that apply to an isolation area (see section 2.1.4).
- In consultation with your herd veterinarian, develop and use drug treatment protocols and follow the instructions properly. Document all treatments that are applied and their outcomes in the herd record.
2.2.3 Implement herd health management protocols
To guide the effective implementation of the herd health management program, a plan is in place outlining the schedule of tasks, as well as the assignment of roles and responsibilities for farm workers. Records are maintained to document the progress and completion of each component.
- Develop Standard Operating Procedures (SOPs) for the implementation of all elements in the herd health management program (see section 2.2.1 and Appendix A: Writing a standard operating procedure).
- Prepare a schedule for herd health management activities, including daily, weekly, monthly and yearly tasks.
- Assign roles and responsibilities for all staff. Review the protocols with all farm workers regularly and provide training as required (see section 2.6).
- Plan in advance for any activities that require outside assistance.
- Record all activities associated with the herd health management program (see section 2.5).
2.2.4 Recognize susceptibility and maintain separation
To prevent the spread of disease within the population of goats on a premises, animals are grouped, based on their current health status and level of immunity, and are managed accordingly. To limit the risk of exposure to disease, the sequencing of all activities is considered.
- Determine the relative susceptibility to infection of goats on the basis of age, immune status, and production status. For example:
- younger animals are more naïve (i.e., have no immunity) than older animals;
- animals in late pregnancy and early lactation, and animals that have been moved or stressed in another way may be more immune-compromised and therefore susceptible to disease;
- the health status and previous exposure to disease of new animals may be different than that of the resident animals in your herd. The animals with less exposure and those with compromised health may be more susceptible to disease; and
- sick animals are more susceptible than healthy animals.
- Avoid placing animals of differing susceptibility or immune status in adjacent pens or the same pasture.
- Handle goats in order of susceptibility, from most susceptible to least.
- Move goats in order of susceptibility, from most susceptible to least.
- Milk goats in order of susceptibility, from most susceptible to least. Milk goats known to be shedding S. aureus last.
- Apply these principles to the use of equipment and tools (see section 2.4.5).
Case Study: Infection Cycle of Johne's Disease
A female kid is born into a herd in which one doe is infected with Johne's disease. Although the kid's dam is not infected, the infected doe purchased the year before is shedding the Johne's bacteria in its manure. The manure has contaminated the bedding and the kid's dam has laid on the dirty bedding so that the bacteria are now contaminating the teats. When the kid first nurses, it consumes colostrum containing the bacteria. During its early life, it again inadvertently ingests bacteria from the teats, bedding, and feed contaminated from goats putting their dirty feet in the feeders. By the time the kid is 3 months of age, it is infected with Johne's, and by the time it is 12 months of age it is shedding Johne's bacteria in its manure. It gives birth at 18 months of age and its own kids become infected from bacteria shed in the milk and manure. At 2 years of age, this animal is showing clinical signs of wasting and is culled to slaughter by the time it is 25 months of age. During the doe's short life, the bacteria shed in its manure and milk has managed to infect another 15 kids.
The producer now wants to focus on eradicating this disease from the farm. With the assistance of the herd veterinarian, a comprehensive program has been developed for the herd. One component of this program is a separate area for kid rearing to prevent exposure to manure from adult goats. This area has dedicated feeders, waterers and equipment. Staff start their daily activities in this area. If they need to return after contact with other animals, they are required to change their clothing and footwear and wash their hands before handling the kids. - If possible, avoid using community pastures, as they present elevated risk of disease transmission. If community pastures are part of your farm management plan, it is recommended to:
- determine whether the health status of other herds using the pastures is compatible with your own;
- limit the time spent on community pastures;
- have an agreement with other members to communicate any changes in health status.
2.2.5 Optimize nutrition and the use of vaccines and other biologics
Nutrition and vaccination plans are in place that address the specific needs of the herd and contribute to enhanced immunity and herd health.
- Develop a nutrition plan for each production stage. Consult with a nutritionist, as needed.
- Analyze forages routinely post-harvest to determine whether they are of sufficient quality and nutritional value.
- Evaluate the total mixed ration (TMR) for particle size and overall nutrients. Use the analysis to validate that the custom supplement is formulated correctly.
- Conduct regular evaluation of body condition to assess whether the nutritional needs of the goats are being adequately met. This is especially important during peak times of the production cycle. More information is available through Langston University.
- Walk all areas of the farm that could be accessible to the herd and identify and eradicate any plants that are potentially toxic to goats. Potentially toxic plants include:
- Monkshood
- Milkweeds
- Foxglove
- Hellebores
- Hydrangea (wilted leaves contain cyanide)
- Black Walnut
- Pokeweed
- Cherries (wilted leaves contain cyanide)
- Rhododendrons and Azaleas
- Nightshades
- Yew
- False Hellebores
More information on toxic plants, including a comprehensive image library, is available through Cornell University. If you suspect your goats may have been exposed to a toxic plant, contact your herd veterinarian for additional guidance.
- Ensure newborns receive a sufficient volume of good quality colostrum shortly after birth. If hand feeding, 5% of body weight soon after birth (50 ml per kilogram or 1 ounce per pound of body weight) and 20% of body weight within the first 24 hours of life is recommended; the first feeding should occur within 4 hours of birth.
- Heat treat colostrum to reduce risk of transmission of some infectious agents (e.g., caprine arthritis encephalitis virus) to newborns. This involves heating the product for 1 hour at 56 degrees Celsius. Temperature control is important, as overheating the product can damage the quality of the colostrum, but underheating will not destroy any potential infectious agents.
- Ensure kids are receiving adequate quantities of milk. Use only clean (i.e., not from a sick animal) pasteurized milk or good quality goat milk replacer. If hand-feeding, make sure feeding equipment is routinely cleaned and disinfected between uses.
- Consider vaccination for the following diseases if they are diseases of concern in your herd:
- Clostridial diseases (e.g., Clostridium perfringens type D or pulpy kidney/enterotoxemia; Clostridium tetani or tetanus; and others)
- Caseous lymphadenitis (Corynebacterium pseudotuberculosis)
- Chlamydial abortion (Chlamydophila abortus)
- Q fever (Coxiella burnetii)
- Rabies, especially in endemic, high-prevalence areas
Vaccines for Goats:
In Canada, no vaccines are approved for use in goats. All vaccines should be administered under the advice and guidance of a veterinarian. Vaccines that are safe in other livestock have been known to cause illness in goats. Vaccines administered to pregnant goats have been known to be associated with an adverse reaction. - Ensure proper administration of vaccinations. This includes appropriate timing of the vaccination during an animal's lifetime and the required boosters. To maximize herd immunity, it is necessary to adhere to the vaccination protocol.
2.2.6 Control movement of animals within the production area
When animals are moved within the production area, the movement is planned in advance to reduce the risk of disease exposure and spread to susceptible animals.
Animals should remain with individuals of the same age and health status within the production cycle, and the group should be moved as a unit (e.g., nursing kids are weaned at the same time and moved as a group). When moving to a new location in the barn, they should move as a group following the principle of all-in, all-out movement. With all-in, all-out movement, all the animals within common-risk groupings are moved together from location to location. This ensures that animals of similar health status and disease susceptibility stay together and are not exposed to animals that may be a potential disease risk. It also creates the opportunity for appropriate premises management practices to occur prior to entry to or after exit from a specific area.
To limit the risk of disease transmission among goats in your herd when moving through your farm facility and along its pathways for routine activities (e.g., to a handling area, to the milking parlour and back), consider the following:
- Study and identify pathways and routes according to their relative risks to healthy animals (see section 1.4).
- Avoid routes and pathways that take healthy goats past or adjacent to sick or isolated goats of unknown health status and vice versa.
- Plan movement in the following sequence: from young to older stock, from healthy to sick, and from higher to lower risk of infection.
2.2.7 Manage feed, water and bedding
Management practices are in place to ensure that feed, water, and bedding are of sufficient quantity and quality, and are free from any potential contamination.
Feed
- Purchase feed from suppliers who produce quality feeds that are labelled and comply with regulations for feed designated for ruminant feeding. Ensure that it is transported in a clean carrier.
- Test the product and supplement the ration, as required, to ensure optimal production and good health among your herd (see section 2.2.5).
- Take feed and forage samples from each batch. Label and store them to allow testing at a later date for quality and for the presence or absence of toxins, if necessary.
- Store feed in a secure, clean facility that limits degradation of feed and prevents access by wildlife, rodents, pests, dogs and cats; when spoiled feed is discovered, remove the feed and address the cause of the degradation and/or contamination.
- Design and position feeders to prevent fecal and other contamination by goats while in use. If feeders become contaminated, remove and dispose of the feed, and then clean and disinfect the feeders before use.
Water
- Ensure that water provided to the herd is safe for livestock consumption. Ideally, the water should be from a municipal or well source and not an open water source, such as a pond.
- Test water at the source at least annually to ensure it is safe for livestock consumption. Guidelines are available through the following links:
- Design and position water bowls, troughs and other waterers to prevent fecal and other contamination by goats while in use.
- Dispose of contaminated water when found and clean and disinfect the waterer(s) before the next use.
- Discuss the implications of any change in water source with your herd veterinarian or other advisor prior to the change (e.g., from well to municipal source and, in some cases, from dug to bored well).
Bedding
- Purchase bedding from a reputable supplier.
- Ensure that bedding is delivered in a clean, uncontaminated condition and is stored in a manner that preserves its condition for use.
- Remove and replace soiled bedding regularly. The frequency should be based on a risk assessment of the pens and housing facilities used by your herd. Consider factors such as the density of animals, the level of contamination and the disease status of the animals. Add clean straw or bedding regularly when using dry pack bedding.
- Remove bedding from hospital and isolation pens and replace on a daily basis or more often, if required; move it to an area that does not have animal access.
2.3 Key area of concern 3: Facility management and access controls
Target Outcome: Management of farm access, facilities, and identified risk areas limits disease introduction and spread on-farm and enables the implementation of biosecurity practices.
2.3.1 Zoning and facility design
Farm facilities are zoned and identified risk areas are determined so that animals, people, and vehicles can move around the farm without unnecessary contact with the herd, and apply the appropriate biosecurity practices.
- Follow the process described in Create a farm map and identify risk areas (section 1.4) and prepare a set of drawings of your farm and your barn area(s); decide where your CAZ and RAZ will be and include them in your biosecurity plan.
- Using the diagram of the barn and confinement areas, document specific pens, work areas and pathways that are of high risk and note the activities and movements that make them high risk.
- Decide where changes in pen allocations and modifications to the goat movement pathways could be made to reduce the higher-risk situations.
- Decide where cleaning and disinfection of instruments, tools and equipment can be done and where dedicated instruments, tools and equipment should be considered.
- Identify controlled access points (CAPs) between areas of differing risk where biosecurity practices need to be followed, especially with respect to cleaning and disinfection of instruments, tools and equipment, hand washing, changes of clothing and footwear, and other practices.
- Mark locations where physical barriers and/or signage are needed to ensure that unintended access to higher-risk areas is limited.
- Prepare and locate signage:
- indicate high security areas
- identify areas not to be used as pathways or for access
- outline movement for pathways
- consider colour coding for different areas and risks
2.3.2 Perimeter and interior fencing
Fencing is used to maintain separation between goats and other animals on the farm, and between the herd and livestock on adjacent farms. Fencing also serves to separate certain goats from the rest of the herd under pre-planned circumstances.
- Install and maintain perimeter fencing to ensure that goats are not released into uncontrolled areas (outside the CAZ) and to restrict interaction with wildlife.
- Install and maintain interior fencing that is appropriate for its biosecurity purpose. For example, when pasturing animals of differing health status in adjoining pastures, consider a buffer zone between the pastures.
2.3.3 Cleaning and disinfection of facilities and on-farm equipment
Cleaning and disinfection is conducted prior to and after use, as well as in the routine maintenance of equipment and facilities. It is focused on the facilities that house the herd, and the tools, and equipment used to manage the herd and handle individual goats.
Ideally, facility surfaces, tools, equipment and vehicles are cleaned and, when required, disinfected, on a schedule determined by a risk assessment process carried out in advance.
Table 3: Five-step Cleaning and Disinfection Process
The five steps in the cleaning and disinfection process are explained in the table. When possible, these steps should be followed.
1. De-bulk: Remove all visible contamination from surfaces of your facilities, tools, equipment and vehicles. This typically involves using a combination of machinery, shovels, brooms and water.
2. Wash: When the area looks clean, it should be washed using a soap or detergent. This should include physical scrubbing; for facility surfaces, tools, equipment and vehicles, use brushes or a pressure washer.
3. Rinse: After washing, all soap and residue should be removed by thorough rinsing.
4. Disinfect: For facilities, tools, equipment and vehicles, the area should be soaked with an approved disinfectant that is appropriate for the infectious agents you are targeting. The disinfectant should be made at the correct concentration and left on the area to be disinfected for the prescribed length of time.
5. Rinse: When required, all traces of the disinfectant should be rinsed away and the area left to dry.
Meat goat farms, and others who might be concerned about the biosecurity risk in pasture areas, can employ a downtime cycle between uses that is long enough to allow infectious agents to be reduced by natural causes.
Choosing a disinfectant can be a complex process. All disinfectants have strengths and weaknesses. When choosing a disinfectant, consider the following to determine if it is appropriate for the intended use:
- what organisms it is effective against (i.e., what type of bacteria, fungi, viruses, coccidial oocysts, protozoa, will it kill ["-cidal"] or inhibit ["-static"]?)
- surfaces and materials on which it can be used
- activity in water of different temperatures or mineral contents
- compatibility with other disinfectants and soaps
- contact time
- animal safety, including use on feeding equipment or watering systems
- human safety
- residual activity
- environmental impact, including method of disposal
Consult your herd veterinarian or industry representative for recommendations. Always follow the manufacturer's directions and respect the product expiry date.
Equipment:
Ideally, equipment is designated to specific areas of common risk, such as the isolation area or milking parlour, and is not used in other areas of the operation; or it is designated to certain tasks, such as a loader bucket for handling manure and/or buckets, forks and shovels for bedding and feed, and is not used for other purposes. This simplifies the cleaning and disinfection process by ensuring that contamination does not occur. When it is not possible to separate equipment by risk zones or activity, you can:
- Create a SOP for cleaning and disinfection, specifying when (how frequently, or after which uses) and how (by what methods and using what equipment) it is done. The default plan would be to clean and disinfect, using the five-step process described above, between each use and different uses.
- Ensure that those responsible for cleaning and disinfection know what type of soap or detergent and disinfectant solutions are required for each task and how they work, including contact time and recommended rinsing and drying requirements for maximum effectiveness.
- Clean and disinfect feeders and waterers regularly, based on use and experience, and whenever contamination with manure, urine and/or other potentially-contaminated materials occurs, and whenever they are being prepared for use by other goats of differing health status.
- Store equipment that has been cleaned and disinfected in a clean environment.
Facility:
- Set an appropriate interval for cleaning. For example, cleaning and disinfection should be completed before or after a significant management event such as milking, clipping or shearing, removal of manure and/or bedding.
- Regularly scrape and clean pathways used by goats, depending on frequency of use, and disinfect based on a risk assessment. For example, pathways should be fully cleaned and, where possible, disinfected when used by goats of different disease status and/or susceptibility that pose a risk of disease transmission. Pathways that are in regular use should be cleared of manure and other debris at a frequency that prevents build-up of these materials and subsequent spread by the animals around the facility and to their pens and other housing areas.
- Pay special attention to milking parlours and holding areas. Milk goats in groups according to disease status and risk. Milk lower-risk goats first (e.g., first fresheners, goats which have undergone disease testing) and higher-risk goats last. After milking, equipment, stands, stanchions, holding areas, and alleyways should be cleaned and disinfected. Water bowls and feeders present in the parlour should also be cleaned and, where appropriate, disinfected. In some provinces, standards have been established for cleaning milking equipment. Consult your provincial government and follow the provincial recommendations.
- Clean and, where possible, disinfect pens and other containment areas based on use and a risk assessment. Pens and areas should be cleaned and disinfected whenever deadstock, aborted fetuses and placentas are discovered, and after each use for identified risk areas including the isolation areas, kidding areas and hospital pen. Some other considerations that will influence your risk management decisions to disinfect are the density of animals, level of contamination, and health status of animals.
- Ensure complete removal of visible organic material before disinfection.
Vehicles:
- Restrict access of off-farm vehicles. Vehicles that travel from farm to farm should not enter the CAZ unless they have been cleaned and disinfected. Animal transportation vehicles should be loaded and unloaded at the periphery of the CAZ and animals can then be led to the isolation area (see section 1.4). Feed trucks and milk trucks should also be unloaded or loaded without entering the CAZ. In all these cases, the perimeter of the CAZ can be designed to provide a controlled access point for each of these purposes so that efficient operation can be maintained. These controlled access points should be managed in such a way that any contamination that may be present on the undercarriage or exterior of the vehicle will not be deposited in the CAZ.
- Clean and disinfect the interior of the trailers or boxes of vehicles used to transport animals. Animal transportation vehicles that travel from farm to farm should be cleaned and disinfected before their arrival. Livestock vehicles that have not been cleaned and disinfected prior to arrival contain high-risk bedding and manure. Determine their previous use(s), inspect the trailers or boxes and, based on a risk assessment, accept or refuse their use for animal transport.
- Ensure that trailers or boxes that are used to transport feed or silage have not been used for any purpose that presents a biosecurity risk to your herd and that they have been suitably cleaned before use. Inspect the trailers or boxes upon arrival and, based on a risk assessment, accept or refuse the shipment.
2.3.4 Facility maintenance
Well-maintained facilities are less likely to harbour potentially infected material, are more easily cleaned, and are less accessible to rodents and other pests.
- Keep walls, ceilings and floors in good repair so that access by pests and rodents is limited, build-up of organic material is reduced, and cleaning can be completed more effectively.
- Keep a notebook handy to record where repairs are required, so that they can be scheduled and completed in a timely manner.
- Use non-porous materials and face or cover them with smooth materials when building, remodelling, repairing and/or resurfacing any facilities. These surfaces reduce the build-up of organic material in areas accessible to goats, and can be cleaned more effectively.
- Ensure that there are no hanging electrical wires or loose wall panels anywhere in the production area that goats can bite or chew.
- Maintain windows and screens in good repair to restrict access by pests and rodents, and ensure a well-lit environment in barns and other facilities.
- Ensure that ventilation shafts are not obstructed and/or plugged.
- Consider biosecurity when renovating existing facilities or designing a new facility.
2.3.5 Management of deadstock, aborted fetuses and placentas
The herd does not have contact with deadstock, aborted fetuses and placentas. Deadstock, aborted fetuses and placentas are high disease-contamination risks for many common infectious diseases.
- Remove deadstock, aborted fetuses and placentas immediately from contact with the herd and with working/guardian animals, wildlife, other predators, pests, cats and dogs.
- Consider a post-mortem or submission of tissue samples to a veterinary diagnostic laboratory to investigate the cause of death. Discuss the options with your herd veterinarian.
- Ensure that disposal methods comply with municipal and provincial regulations.
- When a disposal service is used, prior to pick-up, secure deadstock, aborted fetuses and placentas in a holding unit or area away from the active production area.
- Prevent access to onsite disposal of deadstock, aborted fetuses and placentas by the herd, other livestock on the premises, guardian animals, cats and dogs.
- Cover deadstock, aborted fetuses and placentas with a suitable material (e.g., shavings) to minimize access by flies.
2.3.6 Management of manure
Herd contact with manure is minimized. Manure is a high disease-contamination risk for most common diseases.
- Remove manure on a regular schedule, based on risk and the frequency of use of the pathways, pens and other housing areas:
- Take care not to contaminate goats of differing disease status with manure being moved
- Clean up spills immediately
- Move manure directly to a secure storage facility away from the active production area. Design the manure storage facility to eliminate runoff of urine and/or other fluids into the production area.
- Ensure that disposal and storage methods, at a minimum, adhere to municipal and provincial regulations.
- Clean and disinfect equipment used to collect and move manure after use and, if possible, dedicate it to that purpose.
- Spread manure on pasture only when it is properly composted. Ensure a sufficient period of time between manure spread and use by pasturing goats and/or other livestock.
2.3.7 Management of pests, wildlife and dogs and cats
Pests, wildlife, dogs and cats are excluded from the production area(s) whenever possible. They may be a high-risk source of contamination for certain common diseases.
Table 4: Management of Pests, Wildlife, Dogs and Cats
This table describes the risks associated with pests, wildlife, dogs and cats and provides some recommendations to reduce the associated disease transmission risks.
| Agent | Nature of Risk | Proactive Tactics |
|---|---|---|
| Pests (e.g., rodents, flies, other insects | Direct transmission of disease Mechanical transmission following contact with animals, manure, placentas and other infectious material Indirect transmission through contamination of feed, water or bedding |
Use pest control programs; follow manufacturers' recommendations if using a chemical product Maintain facilities to limit access, including screening over vents and openings in barns and other buildings Store feed and bedding in a secure location. NOTE: Prevent goats' access to rodenticides, pesticides and other control substances |
| Cats and dogs (pets and working animals) | Direct transmission of disease (e.g. rabies, some tapeworms, Coxiella burnetii) Indirect transmission of disease by contamination of feed, water and bedding (e.g. toxoplasmosis) |
Prevent contact with herd whenever possible (unless guardian animal) De-worm dogs with a veterinary-approved product to eliminate tapeworms Store feed and bedding in secure location If goat meat or offal is to be fed to dogs, cook thoroughly or freeze for one week prior to feeding to eliminate tapeworm cysts |
| Predators | Direct transmission of disease (e.g., rabies) |
Investigate all attacks to determine predator type Use guardian animals Use predator repellents Install and maintain perimeter fencing Consider vaccinating the herd against rabies |
| Wildlife (including birds) | Direct transmission of disease (e.g., rabies) Indirect transmission of disease through contamination of pastures, feed or water (e.g., leptospirosis) |
Use guardian animals Maintain facilities to limit access including screening over vents and other openings in barns and other buildings and blocking rafters Consider vaccinating the herd against rabies Respect any conservation regulations concerning the protection of endangered or threatened species; check with your local authorities |
2.4 Key area of concern 4: Movement of people, vehicles and equipment
Target Outcome: Movement and activities of workers, visitors, and service providers, and their vehicles and equipment do not compromise animal and human health.
2.4.1 Access management for farm employees
Farm workers should understand the biosecurity practices that pertain to working on the farm. They are aware and comply with any changes to the biosecurity plan and practices.
- Ensure that all farm workers are aware of the biosecurity practices on your farm and are prepared to implement them.
- Designate a parking area for farm workers. Require them to sign in using an attendance record or the farm's log book.
- Review your daily work plan upon arrival and discuss all biosecurity practices and any materials that will be needed. Discuss problem areas and any special or additional practices that will need to be followed.
- Schedule regular training sessions for all farm employees and confirm their understanding of the practices. Carry out additional training sessions if a practice changes or if a new biosecurity practice is added to the farm plan.
2.4.2 Access management for visitors and service providers
Visitors and service providers access only areas of the farm that are necessary. They are aware in advance of the biosecurity practices that will apply prior to their visit and come prepared.
- Prepare and post signage at the farm gate and at the entrance to the CAZ, notifying service providers and visitors that your farm follows a biosecurity plan. Provide a name and number for them to contact upon arrival for further instruction.
- Post protocols describing the biosecurity practices that are required in each zone and identified risk area.
- Plan all farm visits in advance. Ensure that you and the service provider or visitor both understand and agree to the schedule and biosecurity requirements for the visit.
- Complete a risk assessment for each person planning to visit the farm and enter the production area. Use the following questions as part of your risk assessment:
- Have they visited other farms before arriving at yours?
- Did they interact with goats or other livestock when there?
- Which areas of your farm do they need to access?
- What is the extent of their interaction with your goats while on your farm?
- Ensure that you and all employees are aware of the arrival of all service providers and visitors.
- Meet all farm visitors outside the CAZ when they arrive and record their visit in your visitors' log (see Appendix B: Sample visitor log). Discuss the layout of your farm, which areas they are permitted to access, and what biosecurity practices need to be applied in that location.
- Outline the restricted areas that the service provider or visitor cannot access.
- Permit contact with animals only when necessary.
- Explain the location and use of personal protective equipment (PPE), and how to put it on and remove it (see section 2.4.3).
- Provide garbage cans with sealable lids at controlled access points and elsewhere around your farm and ensure that service providers and visitors use them to discard disposable gloves, boot covers, and any potentially-contaminated materials.
2.4.3 Clothing and footwear
Everyone who enters, and works on or visits, the farm wears farm-specific clothing and footwear. Clothing and footwear will be changed when moving between farm zones and when entering identified risk areas of the premises.
- Require that all farm workers have appropriate, clean, farm-specific outerwear and footwear and that they wear them whenever they enter the CAZ.
- Request that all service providers and visitors bring clean and appropriate outerwear and footwear for use while on your farm and that they wear them when crossing into the CAZ.
- Provide clean clothing and footwear or suitable disposable clothing and footwear for farm workers, service providers and visitors who need to enter the RAZ and/or work in the identified risk areas.
- Maintain an extra supply of clean outerwear and footwear and/or disposable outerwear and footwear for use by farm workers, service suppliers and visitors when necessary (i.e., their own attire gets soiled, or they do not bring sufficient clothing for the visit).
- Post protocols to explain the use of protective clothing, footwear and gloves at each point of entry to the CAZ, transition point between the zones and identified risk area.
2.4.4 Hand washing and personal protective equipment
All who enter and work on, or visit the farm wash and/or sanitize their hands upon entry and exit, when moving between farm zones, and when approaching or leaving certain identified risk areas of the premises. The appropriate personal protective equipment is used for each activity.
- Prepare and post a protocol that explains the proper method for hand washing on your farm. It should include all of the following steps:
- wet hands with warm water;
- apply a liquid or foam soap;
- vigorously lather for a minimum of 15 seconds, taking care to remove all visible organic matter;
- thoroughly rinse using a rubbing motion;
- blot dry with a paper towel;
- use the paper towel to turn the tap off to prevent recontamination.
Contact your local public health unit for additional resources on hand washing. - Require hand washing or sanitization and the use of appropriate personal protective equipment (PPE) upon entry into and exit from the CAZ, RAZ and identified risk areas. Hands should also be washed or sanitized before and after any contact with goats, especially those that are diseased or of unknown health status.
- Require hand washing following contact with any potentially-contaminated material, such as deadstock, aborted fetuses, placentas or manure.
- Provide hand washing stations at the entry point to the CAZ and the RAZ and each identified risk area. If water is unavailable, provide an alcohol-based hand sanitizer (60%) and a supply of single-use, moist towelettes at these locations.
- Provide disposable gloves for use by all visitors who will come in contact with your goats and with potentially infectious materials and surfaces. As previously mentioned, gloves should be single-use and do not replace hand washing.
- Provide N95 fitted masks for family members, farm workers, service providers and visitors for use when dealing with high-risk materials (e.g., aborted fetuses, placentas, deadstock and manure) and when an aerosol-transmitted zoonotic disease (e.g., Q fever) is possibly present on your farm.
- Be aware of all workplace safety regulations for your staff and ensure they are followed.
Case Study:
Using hand washing and personal protective equipment to reduce the risk of Q-fever transmission
A dairy goat operation is experiencing some abortions in the yearling doelings purchased last month. The owner contacts the veterinarian regarding this issue. The veterinarian arrives wearing newly donned coveralls, and he disinfected his hands and boots. He is greeted by the owner and her young daughter. She indicates that the kidding doelings are in a separate wing of the barn, isolated from the main herd. The veterinarian identifies an opportunity to set up an identified risk area which may limit the spread of the infection. He also informs the owner of the zoonotic risks associated with infectious abortion diseases (e.g., Chlamydophila or Q fever). He advises that the daughter should stay out of the barn while the abortions are occurring.
The owner had previously obtained personal protective equipment (PPE) in case of an outbreak of an infectious disease, including plastic boots, gloves and coveralls, as well as N95 fitted masks to prevent inhalation of the abortion agent, which may cause illness. The owner and the veterinarian choose to use the outside entrance to prevent having to return through the main barn. She and the veterinarian change into the PPE, leaving outer clothing outside the door. The veterinarian takes samples of the aborted fetuses and placentas, and the remaining tissues are placed into a green plastic bag and secured. The veterinarian sets up a protocol to deal with the outbreak and a plan to prevent the spread of the disease agent. He tells the owner, "it is fortunate that you kept these doelings isolated from your main herd."
When leaving the wing, the PPE is removed and placed into a plastic bag either for disposal (masks, gloves, plastic boots) or laundering (coveralls). The aborted materials are placed into a sealed garbage container used for small deadstock management. Hands and arms are then washed using the disinfectant soap. The samples for the diagnostic laboratory are placed inside another bag to prevent contamination of the truck. The owner decides that the most prudent action is to place a "No Entry" biosecurity sign on both doors. She also posts a sign on the outer door outlining the protocol for putting on and removing the PPE. Because of these precautions, the infection did not spread and she and her family remain healthy.
2.4.5 Movement control of equipment and tools and vehicles
In identified risk areas or for high-risk activities, the farm's equipment, tools and vehicles are dedicated to one activity or area. The farm's service equipment, tools and vehicles are cleaned and disinfected between uses. Equipment, tools and vehicles that are brought onto the farm are moved into a control area only if necessary. Service equipment, tools and vehicles that are brought onto the farm are cleaned and disinfected before arrival and, if they are used, they are cleaned and disinfected between uses.
- Ideally, purchase and use a dedicated set of equipment (shovels, forks, scrapers, buckets, etc.) for use in each of the following areas:
- isolation area for new arrivals or returning animals
- isolation areas for sick animals
- kidding pens
- manure and soiled bedding handling
- deadstock management
- feed management
- clean bedding management
- Ensure that your biosecurity plan includes the use and maintenance of this dedicated equipment in practices designated for these areas and activities.
- If dedicated equipment for identified risk areas or individual activities is not feasible in your operation, use the five-step cleaning and disinfection process to clean and disinfect equipment and tools after each use and when moving between zones (see Section 2.3.3).
- Purchase your own equipment whenever feasible and inform service providers that it is to be used instead of equipment that is brought onto your farm.
- For equipment brought onto your farm (e.g., for custom work), inspect it before use and insist that it be cleaned and disinfected before use.
- Replace off-farm vehicles with farm-specific vehicles for high-risk activities, such as movement of animals and transport of manure.
2.5 Key area of concern 5: Monitoring and record keeping
Target Outcome: Information is maintained and used to improve the effectiveness of biosecurity practices. The status of animal health, identification, and inputs may be verified by record review.
2.5.1 Herd health records
Animals are observed on a daily basis and the findings are documented in the herd health record. Health records are reviewed regularly to detect any changes in animal health and production over time and to initiate the necessary response.
- Maintain herd health records that correspond with your herd health management program (see section 2.2.1). Herd health records should include, but are not limited to:
- Production records: Documented information will be specific to the farm's operation and may include milk production and component testing, kid production, kid weights, or volume, weight and quality of fibre produced. Frequency of recording may be daily, weekly, monthly or on a cyclical basis, depending on the data available;
- Health monitoring: Observations of clinical signs and the progress of any disease from earliest stages to resolution;
- Disease occurrences and interventions: Surveillance for diseases of concern at the farm level; examples include number of cases of pneumonia, number of animals culled for poor body condition, and number of cases of diarrhea in neonatal kids;
- Veterinary care: Treatments discussed with and administered by or under the guidance of your herd veterinarian, including prescription, treatment, withdrawal times and results of the treatment;
- Disease specific programs and control: Related to diseases being actively managed on your farm, including diseases of concern and any industry or regional programs.
- Document this information, along with the date and the name of the person who made the entry, in a manual log book or other permanent record. Computerized records may provide more detailed and current analysis of the data. Hand-held devices may be valuable to efficiently document health and production events.
- Review herd health records with your farm workers when preparing their daily work plan. Determine whether any additional biosecurity practices are needed and ensure that appropriate personal protective equipment (PPE) and cleaning and disinfection supplies are available.
- Review herd health records with your herd veterinarian on a regular basis, and discuss and implement adjustments to practices, if needed.
- Prepare cumulative, herd-wide summaries of the herd health records and review them annually. Use this information to establish new goals and implement changes to your herd health management program, if needed.
- Maintain a summary of the herd health management program and your herd health and disease experience so that it can be used in discussions with potential purchasers.
2.5.1.1 Individual animal records
Records are maintained for each animal to document data on health, productivity and movement throughout the individual's lifetime.
- Maintain individual animal records that include:
- Signalment
- Date of birth, including identification of littermates
- Dam information including herd of origin, important medical history, date of birth
- Sire information including herd of origin, important medical history, date of birth, type of breeding (natural or AI)
- Rearing history, including origin if born off-farm, source of colostrum, dam-reared or artificial rearing
- Production history
- Milk production
- Kid production, including number of kids per birth, kid identification and growth
- Health history
- Abnormal kidding events (if female)
- Body condition information
- Vaccination events
- Preventive treatment events (i.e., deworming, coccidiostats)
- Diagnoses of abnormal health events (e.g., pregnancy toxemia, pneumonia), including treatments and outcomes. Veterinary medical records are included.
- Test results of disease testing (e.g., CAE tests)
- Date and reason of death, including necropsy results
- Date and reason of cull
- Travel history
- Shows attended, dates of departure and return
- Off-farm for other reasons (e.g., semen collection)
- All activities and risk exposures recorded, including other livestock and transportation
- Isolation history
- If isolated for any reason (e.g., illness, abnormal test results, departure and return to the farm), record time in isolation and all test results
- Signalment
In many cases, these individual records may be subcomponents of your overall herd health records.
2.5.1.2 Identification and traceability
Each animal is identified using industry-standard identification, which links each animal with health and production-related data and tracks movement, both on and off the farm, throughout an animal's lifetime.
- Identify each animal using an industry-standard method of identification, such as a Canadian National Goat Federation (CNGF)-recommended identification tag.
- Link the animal's identification to its individual health and production data.
- Use the animal's identification to track all movement, both on and off of the farm, throughout its lifetime.
2.5.2 Farm management records
Records for farm management activities are maintained and reviewed regularly with animal health records to assess their effectiveness in the biosecurity plan.
- Maintain farm management records, which may include but are not limited to:
- farm worker and visitor logs;
- cleaning and disinfection practices;
- manure and deadstock disposal;
- biosecurity training and communication initiatives.
- Document the date, the name of the person who completed the activity, and the areas of the farm involved, if relevant, for each activity.
- Review these records in conjunction with your herd health records to determine whether any farm management activities may be related, positively or negatively, to changes in animal health. Use this assessment to make any adjustments to your farm management practices.
2.5.3 Input records
Information on the source and quality for each purchase of feed, bedding, and any other input is maintained and may be used as a reference if related health issues are suspected.
- Record purchases and deliveries of feed, forages and commercially-available supplements, including date, source or supplier, type, quantity, quality and means of delivery.
- Take a sample of forages and identify it with the record of purchase and delivery above. Test the sample and enter the quality and nutritional value into the record. Provide the test results for formulation of additional nutrients and to balance rations (see sections 2.2.5 and 2.2.7).
- Document the dates of use for each ration, as well as the quantity and the goats to which it was fed.
- Record purchases and deliveries of bedding, including date, source or supplier, type, quantity, quality and means of delivery.
- Record water testing results.
2.6 Key area of concern 6: Communications and training
Target Outcome: Everyone who enters the farm is knowledgeable and complies with current farm biosecurity practices.
2.6.1 Producer leadership
Producers take the responsibility for establishing their farm's biosecurity plan and educating their family members and farm workers. Service providers and visitors understand the farm's plan and abide by its requirements.
- Take the opportunity to discuss your biosecurity plan with all family members, farm workers and service providers frequently so that the plan is seen as a natural part of life on your farm.
- Meet with visitors when you are planning their visit to discuss your biosecurity plan and how it will apply to their activities on-farm.
- Review your biosecurity plan with all of your service providers, either in group discussions or in one-on-one sessions. Clearly explain the intended outcomes of your biosecurity practices and what activities you expect each service supplier to undertake in preparation for a visit to your farm, while they are on your farm, and when they are preparing to leave your farm. Ideally, work with your service suppliers to modify their biosecurity plans to suit your required outcomes.
- Make sure that you adhere to all required biosecurity practices during your activities on the farm. Use these opportunities to demonstrate proper practices to others.
2.6.2 Communication with farm workers, family members, service providers and visitors
Producers communicate the requirements of their biosecurity plans to farm workers, family members, service providers and visitors.
- Choose the method(s) of communication that are suitable to your farm. These may include:
- Discussions in a group setting with all workers together; request feedback on practices and revise protocols, if appropriate, to incorporate the feedback
- Individual discussions in scheduled meetings or on-the-job
- Develop and use both verbal and written material.
- Prepare signage with specific instructions for selected areas of the farm and production areas.
- Maintain records for the application of biosecurity practices in each work area, and use the results for feedback to family, farm workers and service providers.
2.6.3 Training and education
Educating farm workers, family members, service providers and visitors is undertaken whenever practices are added or changed; they are explained to visitors and service providers in advance of their arrival.
- Plan and carry out regularly-scheduled training sessions with all family members and farm workers, both one-on-one and in group sessions; identify areas that require additional training and verify the participants' knowledge during each session.
- Hold training sessions whenever a practice is changed or when a new practice is added to your biosecurity plan.
- Include demonstrations of best practices in training sessions and on the job.
- Monitor the activities of family members and farm workers during their work on the farm. Commend good performance and address any gaps identified in knowledge and ability.
- Explain all applicable biosecurity practices to visitors and service providers. Provide the appropriate training and resource material, as needed.
2.6.4 Performance effectiveness of the biosecurity plan
The effectiveness of the biosecurity plan is measured by the adoption of its biosecurity practices and their integration into daily routines; improvements to the farm's biosecurity plan are designed and implemented.
- Review key health performance indicators contained in the herd health records (see section 2.5) during and following implementation of your biosecurity plan and as changes to the plan are made.
- Discuss your performance with your herd veterinarian and other advisors and adjust your plan, as needed, depending on their analysis.
- Meet with your family members and farm workers at least twice yearly to discuss the usefulness and effectiveness of each of the practices in your biosecurity plan. Measure the usefulness and effectiveness of the practices by the results and changes they see following implementation.
- Review education and training sessions after each session to identify improvements that can be made.
3 Glossary of Terms
The first occurrence of each term in the glossary has been identified in the document with bold text.
- Accredited:
- Approved or recognized as meeting a prescribed standard
- Acute:
- Rapid onset or short duration
- Anteroom:
- A physical space within the production area or barn where there is a transition zone that defines a dirty and clean area where boots and clothing can be changed, and hand washing performed
- Anthelmintic:
- An agent, usually a drug, which is destructive to worms (internal parasites)
- Barn:
- A farm building used for storing farm products and sheltering livestock
- Bio-containment:
- Reducing the spread of infectious agents between goat farms or from goat farms to other animal populations
- Bio-exclusion:
- Reducing the introduction of infectious agents to goats on the farm
- Biologics:
- Medical preparations made from living organisms or their products; examples include vaccines, toxoids, serum, and antigens
- Bio-management:
- Reducing the spread of infectious agents among goats within a farm
- Biosecurity:
- A health plan or measures designed to protect a population from transmissible infectious agents
- Biosecurity protocols:
- Those measures specific to a goat operation used to prevent the introduction and the spread of disease within an animal population and from that goat operation
- Cleaning:
- Involves washing with detergent in order to remove all organic matter, and includes both a dry (scraping and brushing) and wet clean
- Commingling:
- Mixing of animals from different farms or groups resulting in direct or close indirect contact among them
- Community pasture:
- A public grazing area shared by more than one producer and not owned by a single producer
- Confinement area:
- Any pen that is used to confine a single goat or a group of goats to temporarily separate them from others in the herd, and/or to allow producers, veterinarians or service providers to work with them away from the rest of the herd
- Controlled access point (CAP):
- A visually-defined entry point through which all traffic (vehicles, people, animals, inputs and equipment) enter a CAZ and a RAZ; specific biosecurity protocols may be in place
- Controlled access zone (CAZ):
- A designated area where biosecurity protocols are in place and monitored and livestock are managed (e.g., a location or primary location); accessible to people, equipment, vehicles and livestock only through a securable (e.g., lockable) controlled access point
- Cria:
- A young camelid (llama, vicuna, or alpaca)
- Cross-contamination:
- The act of mixing a material, especially a material that is potentially infectious, with another material, thereby introducing the risk that a contaminant could be transferred to an animal; for example, infectious agents shed by sick or carrier animals can be transferred from manure to feed by the use of a common bucket or shovel
- Direct contact:
- Any form of close contact in which goats can physically touch one another, including all forms of nose-to-nose contact
- Disease(s) of Concern:
- Those diseases that pose a high risk to the health and productivity of a herd;
can be farm-specific or applicable to an entire region or country - Disinfection:
- The use of a disinfectant, i.e. a chemical that can kill microorganisms, on objects and surfaces after organic material has been cleaned away; disinfectants are not used on live animals
- Dry pack:
- A bedding approach that is formed by adding more bedding on top of the existing bedding to reduce the frequency of manure removal. A bedded pack can stay dry and warm but it is important to clean it out periodically. Urine moves down through the pack, leaving the top relatively dry; however, packs tend to have high bacteria counts if insufficient bedding is not added daily.
- Endemic:
- Continued presence of a disease in a specific population or area
- Family members:
- Any family members who work on the farm, whether they live there or not
- Farm worker:
- A person who works on the goat operation; may include family members
- Goat operations:
- All of the activities involved in raising goats and working with goat products, including meat, dairy and fibre
- Health status:
- Current state of health of the animal or herd, including both its condition and any infectious agents present in the animal or herd
- Heat treatment:
- Procedure used to prepare colostrum for newborn consumption; involves heating the product for 1 hour at 56°C
- Herd of origin:
- Herd within which the animal was born
- Identified risk area:
- Any area on the premises that has an increased likelihood of disease introduction and/or transmission; this may be due to the nature of the activity that occurs in the area and/or the group of animals that are housed within
- Immunity:
- Resistance to infection and/or disease
- Incubation:
- The period of time between exposure to an infectious agent and the onset of clinical signs of disease
- Indirect contact:
- Any form of contact between goats that involves shared contact with inanimate objects (surfaces, equipment, feed, water, and bedding); does not involve any physical contact
- Infectious agent:
- A microbial pathogen that has the potential to cause disease (bacteria, viruses, parasites, fungi, protozoa, and prions); agents may be shed from an infected animal that appears healthy and is either incubating disease, recovering from disease, or is a carrier without symptoms. Routes of shedding include saliva, milk, respiratory secretions, feces, urine, epidermal shedding, and uterine or vaginal discharges. After shedding, infectious agents can persist in the environment and be transmitted indirectly. For example, the tetanus agent can persist for years in the soil and can still be infectious.
- Infectious disease:
- Disease caused by an infectious agent
- Isolation:
- Restricting an animal to a location that is physically separate from other livestock. The purpose of isolating an animal is usually to prevent it from transmitting a disease to another animal or acquiring disease from another animal, either because it is known to be diseased or because its disease status is currently unknown. The location is known as an isolation facility.
- Loading area:
- An area that is designated for the loading and unloading of animals; this is not just the ramp but it also includes any holding area and handling facilities used for this purpose
- Mammal:
- Any warm-blooded vertebrate of the Class Mammalia, the females of which have milk-producing mammary glands for nourishing young. Other common characteristics include a thoracic diaphragm and, typically, a covering of hair or fur. Examples include whales, humans, carnivores, rodents, goats and bats.
- Mortality:
- A measure of the number of deaths in a population
- Necropsy:
- A post-mortem examination to determine the cause of death; may involve only gross examination, or additional sampling and laboratory testing for infectious agents and/or toxins
- Oocyst:
- The development stage of certain protozoan parasites that follows fertilization of the egg. Oocysts have a strong protective covering that allows them to persist in the environment for extended periods of time and withstand many disinfectants.
- Other livestock:
- Animals other than goats that are used for food or fibre production, work, guardian activity and recreation; specifically sheep, cattle (dairy, beef, veal), horses, bison, water buffalo, farmed deer or elk, alpacas, llamas, swine, chickens, turkeys and fowl
- Pastures:
- Fenced areas used for livestock grazing at any time of year; can include multi-use fields (e.g., graze after haying or aftermath feeding)
- Pests and wildlife:
- Includes all non-livestock and non-domestic animals and birds, and insects that may pose a health risk (disease and/or predatory) to the goat herd
- Pets:
- Cats, dogs and any other household pet kept by the farm family, their neighbours and/or staff
- Practice:
- General procedure that is followed by a producer, and not necessarily documented or detailed to the extent of a protocol
- Premises:
- A defined area of land with all accompanying structures
- Prion:
- An infectious agent in the form of an abnormally folded protein; causes normal prion proteins, which are most commonly found in the brain, to misfold
- Protocol:
- Description of a practice or method, usually written in a standard format that applies to a specific activity and has an intended result or outcome
- Protozoan parasite:
- A single-celled organism that can invade and inhabit cells and tissues of other living organisms
- Reportable disease:
- Those diseases that are outlined in the Health of Animals Act and Reportable Diseases Regulations and are usually of significant importance to human or animal health, or to the Canadian economy. Animal owners, veterinarians, and laboratories are required to immediately report the presence of an animal that is contaminated or suspected of being contaminated with one of these diseases to a CFIA district veterinarian. Control or eradication measures will be applied immediately. Some provinces also have a list of reportable diseases and required response actions.
- Restricted access zone (RAZ):
- An area inside the controlled access zone where goats are housed and where access by people or equipment is further restricted
- Sanitizer:
- A product that kills some micro-organisms and is designed to be used on living tissue or food contact surfaces (e.g., hands, in the milk parlour or milk house); also called an antiseptic
- Service provider:
- A person, company or organization that provides goods or services to farms on a professional basis, including feed and feed additives suppliers, veterinarians, hoof trimmers, shearing or combing technicians, live animal transporters, deadstock pickup services, manure management, and many others. The nature of service providers' activities on a farm, especially their closeness to or interaction with the herd, determines the relative risk of disease transmission that they represent.
- Shedding:
- Transmission of an infectious agent from an individual to another individual or to the environment; can occur in the absence of clinical signs (i.e., before the onset of clinical signs, after recovery from disease, or as a carrier)
- Signalment:
- Information relating to an animal's age, sex and breed
- Source herd:
- The herd from which goats, sperm and/or embryos are purchased; may also be the herd of origin
- Standard operating procedure (SOP):
- A written set of explicit instructions for carrying out a specific task
- Susceptible:
- Lacking sufficient resistance or immunity and therefore at higher risk of infection and disease
- Traceability:
- The ability to follow a product through all stages of the supply chain
- Trigger point:
- A threshold data point (e.g., percentage drop in milk production) or significant observation (e.g., cluster of abortions) that leads to further action (e.g., contact herd veterinarian, move goat to isolation area)
- Verified:
- Proven to be truthful or accurate
- Veterinary client patient relationship (VCPR):
- A veterinary client patient relationship (VCPR) requires on-farm visits at least annually and before prescribing and/or selling medications if the animal health situation changes
- Visitor:
- A non-service provider visiting the herd
- Working / guardian animals:
- Includes dogs (e.g., guardian dogs, herding dogs), llamas, donkeys, and horses that have contact with and are used to manage the goats for purposes such as moving the goats, or guarding the goats from predators
- Zoonotic disease:
- An infectious disease that can be transmitted (in some instances, by an insect) from animals, both wild and domestic, to humans or from humans to animals
4 Acknowledgements
Myrna Coombs: Canadian National Goat Federation
Jennifer MacTavish: Canadian National Goat Federation
Dr. Nancy de With: British Columbia Ministry of Agriculture
Laurie Fries: Alberta goat producer, Alberta Goat Breeders Association
Dr. Kathy Parker: Private sector veterinarian
Dr. Jagdish Patel: Alberta Agriculture and Rural Development
Jared Clarke: Saskatchewan meat goat producer, Canadian Meat Goat Association
Mamoon Rashid: Manitoba Agriculture, Food and Rural Initiatives
Anton Slingerland: Ontario dairy goat producer, Ontario Goat
Lloyd Wicks: Ontario dairy goat producer and breeder, Export Ontario
Jennifer O'Rourke-Bullock: Ontario Goat
Dr. Jocelyn Jansen: Ontario Ministry of Agriculture, Food and Rural Affairs
Dr. Paula Menzies: University of Guelph
Dr. Allyson MacDonald: Private sector veterinarian (MacDonald Mobile Veterinary Service), President Small Ruminant Veterinarians of Ontario
Jean-Philippe Jolin: Quebec goat producer, Syndicat des Producteurs de Chèvres du Québec
Virginie Rochet: Sector specialist, Agriculture and Agri-Food Canada
eBiz Professionals: Consultant
Office of Animal Biosecurity: Canadian Food Inspection Agency
Appendix A: Writing a standard operating procedure (SOP)
What is an SOP? "Written procedure prescribed for repetitive use as a practice, in accordance with agreed upon specifications aimed at obtaining a desired outcome."
As part of a herd health program, the herd veterinarian may provide explicit instructions for the producer on specific management procedures or management of certain disease conditions. A well-written SOP will provide objective and thorough instructions that complement a herd health program. They will provide evidence of a valid veterinary client patient relationship, which may include the proper extra-label use of animal health products (ELDU) and appropriate withdrawal times. They may provide information on what to do until the veterinarian arrives or when it is prudent and timely to call the veterinarian.
The structure of an SOP may vary depending on the issue being addressed.
- Numbered sequence:
- This is a list of tasks to be completed in the order that they are to be performed. No decision-making or data input is required.
- Flow chart:
- Requires decision-making or data input at one or more steps. For example, if a temperature is greater than or less than a certain cut-point, the person may be directed to perform different tasks or administer different treatments.
- Additional information:
- Information on what to do if things go wrong, or if an adverse reaction occurs. It may be as simple as "call the vet!" or it may be to highlight a specific risk (e.g., accidental injection of a potentially toxic medication).
- Ancillary documents:
- This may also include records to be kept relevant to the SOP. Some SOPs may require additional documentation, e.g., a feed prescription for ELDU use of medicated feeds.
The SOP consists of:
- Title:
- This should be a succinct and accurate description of the SOP being addressed.
- Rationale for the SOP:
- A short description of the disease or health issue being addressed.
- The SOP:
- In language that is clear and succinct. Depending on the SOP, it may be a numbered sequence or an algorithm. If the latter, indicate clearly what data needs to be assessed and the cut-off points for each decision point. Structure the SOP so that it can be posted in the relevant management area.
- Additional Information:
- This includes (where relevant) appropriate withdrawals for meat and milk; risk to humans or animals; where to seek additional help if a problem arises, etc. It may also include a list of supplies, medications and equipment needed to execute the SOP.
- Ancillary Documents:
- Describe any record-keeping that should be done to ensure that the SOP is being followed properly or to ensure that it is working. List any other documents that might need to be included, e.g., if a feed prescription is required, indicate this.
- Outcome Assessment:
- Indicate here what outcomes should be measured, which would indicate that the SOP was (or was not) being executed successfully or was accomplishing its purpose.
Appendix A was kindly provided by Dr. Paula Menzies.
Appendix B: Sample visitors' log book page
A sample log book page can be found on page 58 of the Livestock On-Farm Biosecurity Information Guide, which was developed by the Ontario Livestock and Poultry Council (OLPC). The Canadian National Goat Federation and the Canadian Food Inspection Agency thank the OLPC for their assistance.
Appendix C: Bibliography
The following lists include the total set of documents that have been selected from the literature review as relevant and important for producers preparing a biosecurity plan based on the Standard.
Initial References
Antonia M., Clavijo, B.M. "Effect of the exploitation system on the appearance of mastitis in goats in two farms in Falcon state, their etiologic agents and antimicrobial resistance. Zootecnia Tropical 20, 383-395 (2002).
Bates, T.W., Thurmond, M.C. & Carpenter, T.E. "Direct and Indirect Contact Rates Among Beef, Dairy, Goat, Sheep, and Swine Herds in Three California Counties, with Reference to Control of Potential Foot-and-Mouth Disease Transmission." American Journal of Veterinary Research 62, 1121-1129 (2001).
Dement, A.I. & Craddock, B.F. "Biosecurity for Sheep and Goat Producers." Texas A&M University.
Ganter, M. "Veterinary Consultancy and Health Schemes in Sheep: Experiences and Reflections from a Local German Outlook." Small Ruminant Research 76, 55-67 (2008).
Ghanem, Y.M. et al. "Prevalence and risk factors of caprine arthritis encephalitis virus infection (CAEV) in Northern Somalia." Small Ruminant Research 85, 142-148 (2009).
Givens, M.D. & Marley, M.S.D. "Infectious Causes of Embryonic and Fetal Mortality." Theriogenology 70, 270-85 (2008).
"Goat MAP: Rules and Guidelines of the Australian Johne's Disease Market Assurance Program for Goats." Animal Health Australia, Goat Industry Council of Australia Inc., Nation Johne's Program 1-67 (2009).
"Goat 2009 Part II: Reference of Goat Health and Marketing Practices in the United States, 2009." Animal and Plant Health Inspection Service (2011).
Howell, S.B. et al. "Prevalence of Anthelmintic Resistance on Sheep And Goat Farms in the Southeastern United States." Journal of The American Veterinary Medical Association 233, 1913-1919 (2008).
Kabagambe, E.K. et al. "Risk Factors for Brucella Seropositivity in Goat Herds in Eastern and Western Uganda." Preventive Veterinary Medicine 52, 91-108 (2001).
Menzies, P.I. "Control of Important Causes of Infectious Abortion in Sheep and Goats." The Veterinary Clinics of North America Food Animal Practice 27, 81-93 (2011).
Menzies, P. & Simard, C. "Ontario Maedi Visna Herd Status Program: Definitions and Protocols Governing the Program and Additional Information." University of Guelph 1-29 (2007).
Merkel, R.C. & Gipson, T.A. "Change in behaviour of goat producers after on-line training in herd health practices." Small Ruminant Research 98, 31-34 (2011).
Merkel, R. "Introduction to a Meat Goat Quality Assurance Program and HACCP." Langston University.
Mobley, R. & Lyttle-N'guessan, C. "The Herd Health Handbook for Goat Producers: Biosecurity at the Farm Level." Florida A&M University (2009).
Mobley, R. & Lyttle-N'guessan, C. "The Herd Health Handbook for Goat Producers: Food Safety at the Farm Gate: A Holistic Approach to Food Safety and Herd Health." Florida A&M University.
Mobley, R., Lyttle-N'guessan, C. & Peterson, T. "The Herd Health Handbook for Goat Producers: Control of Parasites in Goats." Florida A&M University (2009).
Moore, D.A. et al. "Comparison of Published Recommendations Regarding Biosecurity Practices for Various Production Animal Species and Classes." Journal of the American Veterinary Medical Association 233, 249-56 (2008).
Nöremark, M., Frössling, J. & Lewerin, S.S. "Application of Routines that Contribute to On-Farm Biosecurity as Reported by Swedish Livestock Farmers." Transboundary and Emerging Diseases 57, 225-236 (2010).
Oliveira, C.J.B. et al. "On farm risk factors associated with goat milk quality in Northeast Brazil." Small Ruminant Research 98, 64-69 (2011).
Olson, E.J. et al. "Isolation of an Adenovirus and an Adeno-Associated Virus from Goat Kids with Enteritis." Journal of Veterinary Diagnostic Investigation 16, 461-464 (2004).
"Sheep/Goat Industry Biosecurity Plan." farmbiosecurity.com.au 1-4 (2003).
"Sheep and Goat Industries Biosecurity Plan." Government of Western Australia Department of Agriculture 1-64 (2002).
Thunes, C. & Carpenter, T.E. "Biosecurity Practices and Travel History of Individuals Exhibiting Livestock at the 2005 California State Fair." Journal of the American Veterinary Medical Association 231, 581-5 (2007).
Winter, A.C. "Treatment and Control of Hoof Disorders in Sheep and Goats." The Veterinary Clinics of North America Food Animal Practice 27, 187-192 (2011).
Additional References
Clark, C., Parker, K. & Woods, J. "Sheep and Goat Management in Alberta Health." Alberta Lamb Producers and Alberta Goat Breeders Association 1-262 (2009).
eBiz Professionals Inc. "Beef, Veal, Sheep and Goats: Biosecurity and Emergency Preparedness Gap Analysis." 1-282 (2010) - PDF (3.66 MB)
"Goats and the Smallholder - Some biosecurity tips." Tasmania - Department of Primary Industries, Parks, Water and Environment.
Häusermann, C. et al. "Surveillance and Simulation of Bovine Spongiform Encephalopathy and Scrapie in Small Ruminants in Switzerland." BMC Veterinary Research 6, 1-13 (2010).
Humann-Ziehank, E. & Ganter, M. "Preventive Animal Health in Small Ruminants - Results of an Interdisciplinary Workshop Part 2: Infectious Diseases." Tieraerztliche Umschau 61, 91 (2006).
Kao, R.R. et al. "Disease Dynamics over Very Different Time-Scales: Foot-And-Mouth Disease and Scrapie on the Network of Livestock Movements in the UK." Journal of the Royal Society, Interface 4, 907-916 (2007).
Kitching, R.P. & Hughes, G.J. "Clinical Variation In Foot And Mouth Disease: Sheep And Goats." Revue Scientifique et Technique de L'Office International des Épizooties 21, 505-512 (2002).
Kumar, S., Vihan, V.S. & Deoghare, P.R. "Economic implication of diseases in goats in India with reference to implementation of a health plan calendar." Small Ruminant Research 47, 159-164 (2003).
Olcott, B. "Biosecurity for Meat Goat Producers." Proceedings of the 22nd Annual Goat Field Day, Langston University. 1-17 (2007).
Paton, M. "Cheesy Gland in Sheep and Goats." Government of Western Australia - Department Of Agriculture (2005).
"Recommended code of practice for the care and handling of farm animals - Goats." Canadian Agri-Food Research Council 1-78 (2003).
Reviriego, F.J., Moreno, M.A. & Dominguez, L. "Risk Factors For Brucellosis Seroprevalence Of Sheep And Goat Herds In Spain." Preventive Veterinary Medicine 44, 167-173 (2000).
Seuberlich, T., Heim, D. & Zurbriggen, A. "Atypical Transmissible Spongiform Encephalopathies in Ruminants: A Challenge for Disease Surveillance and Control." Journal Of Veterinary Diagnostic Investigation 22, 823-842 (2010).
White, E.C. "Biosecurity Concerns for Sheep and Goat Herds: A Biosecurity Plan for Small Ruminant Farms." Tufts University 1-2 (2004).
Wrathall, A.E. "Risks of Transmission of Spongiform Encephalopathies by Reproductive Technologies in Domesticated Ruminants." Livestock Production Science 62, 287-316 (2000).
Wrathall, A.E. "Risks of Transmitting Scrapie and Bovine Spongiform Encephalopathy by Semen and Embryos." Revue Scientifique Et Technique - Office International Des Épizooties 16, 240-264 (1997).