The Feeds Regulations, 2024 were published on July 3, 2024, and have replaced the Feeds Regulations, 1983. We are working to update this page and the remaining website to reflect the Feeds Regulations, 2024. As new updates and guidance continue to be released, please refer to the Feed regulatory modernization page for more information.
Purpose
To outline the Canadian Food Inspection Agency (CFIA) policies regarding requirements for the:
- application of the enhanced feed ban statement on animal food labels and documentation;
- application of lot numbers on animal food labels and documentation; and
- retention of records,
and provides guidance on how these policies are to be applied.
Regulatory Authority
Part XIV of the Health of Animals Regulations, "Food for Ruminants, Livestock and Poultry, Rendering Plants, Fertilizers and Fertilizer Supplements", administered by the CFIA, contains several regulatory measures associated with the production of animal food and the feeding of livestock. It includes a prohibition on feeding ("feed ban") of most proteins derived from mammals (defined as "prohibited material" (PM)), to ruminant animals such as cattle, sheep, goats and deer. It also includes requirements for anyone who manufactures, imports, packages, stores, distributes, sells, or advertises for sale animal food for ruminants (and other animal species as specified in section 171.(1) of the Health of Animals Regulations) to keep records (for 10 years) that contain the lot number and any other information used to identify each lot of animal food.
1. Application of the enhanced feed ban statement on labels and documentation
Policy Context and Terminology
In June 2006 the CFIA published regulatory enhancements to the 1997 feed ban which included, in the Health of Animals Regulations, a change to the statement required on labels and documentation for animal food containing prohibited material. On July 12, 2007, these regulatory changes came into effect, and the following statement is now required:
Feeding this product to cattle, sheep, deer or other ruminants is illegal and is subject to fines or other punishment under the Health of Animals Act./Il est interdit d'en nourrir les bœufs, moutons, cerfs et autres ruminants et des amendes ou autres peines sont prévues à cet égard par la Loi sur la santé des animaux.
Within this policy document, this statement is referred to as the "enhanced feed ban statement".
The Feeds Regulations were also amended to require the use of this statement on labels of feeds containing prohibited material. Consequently, this statement is required on labels of products to which the Feeds Regulations apply if they contain prohibited material.
Policy Principles
The 2007 enhanced feed ban includes the removal of Specified Risk Material (SRM) from animal food, pet food, and fertilizer, to further protect animal health and accelerate the eradication of BSE in Canada. This measure is intended to remove potential known infectivity from the feed system and reduce the possibility of exposure; nevertheless, cross contamination of ruminant feeds or incorrect feeding of ruminants with prohibited material may still occur.
As a result, section 170.(1) of the Health of Animals Regulations retains the necessity to have procedures "to prevent the mixing or contamination of the rendering plant product or animal food for ruminants, with prohibited material" (where prohibited material is on the same premises as animal food for ruminants or rendering products that do not contain prohibited material). Consequently:
P.1 For animal food and ingredients for the non-ruminant animals specified in regulationsFootnote 1 that contain prohibited material in their formulation, the enhanced feed ban statement must be used on labels and documentation that relate to such products in order for the labels and documentation to be in compliance with regulatory requirements.
P.2 For animal food and ingredients for the non-ruminant animals specifiedFootnote 1 that do not contain prohibited material in their formulations, the enhanced feed ban statement must not be used on labels and documentation that relate to such products, with the exceptions noted below.
- For animal food and ingredients used in rations for the non-ruminant animals specified that do not contain prohibited material in their formulations but have been in contact with animal food or ingredients that contain prohibited material, such as:
- materials used to flush processing systems or equipment immediately after the production of products containing prohibited material that are recovered, stored or re-used in other products; or
- dust, spillage, reworked and other materials recovered, stored, or re-used in other products in a facility that handles prohibited material,
- For animal food and ingredients for the non-ruminant animals specified that do not contain prohibited material in their formulations but are manufactured in the same processing line or equipment immediately after animal food or ingredients that contain prohibited material, without a validated clean-out (e.g. flush) or other cross-contamination prevention measure employed in between processing runs, the enhanced feed ban statement must be applied on labels and documentation that relate to the animal food.
The Decision Tree diagrammed below illustrates how these principles apply.
Figure 1: Decision Tree - Application of the Enhanced Feed Ban Statement
Click on image for larger view
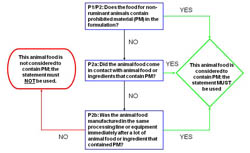
Decision Tree - Application of the Enhanced Feed Ban Statement
Decision Tree - this diagram illustrates how the policy principles outlined in the document apply to the labelling of animal food and ingredients. Specifically, it outlines a step-wise approach to determining when the "enhanced feed ban statement" is to be used on labels and documentation of animal food and ingredients, depending on whether they contain prohibited material or not, or came in contact with prohibited material.
Questions & Answers
The following questions and answers and specific scenarios are provided as guidance on the policy principles, their interpretation and application.
Q. Does the enhanced feed ban statement on labels and documentation have to be in both official languages (English and French)?
A. The required statement can be in either English, French or both languages, provided that it is in the same language(s) as the rest of the label (i.e., a bilingual label must have a bilingual statement).
Q. Does the enhanced feed ban statement have to be applied on labels for rabbit, mink, fox and fish feeds, if they contain prohibited material (PM)?
A. Yes. Feeds for rabbits, mink, foxes and fish (species not identified in section 169 of the Health of Animals Regulations, but to which the Feeds Regulations apply), requires the enhanced feed ban statement on labels, if any feeds for these species contain PM.
Q. Does the entire enhanced feed ban statement have to appear on mixing formulae, mixing sheets and other product documentation to identify the presence of PM in a animal food? If not, what alternative means of identification are acceptable?
A. No. Given that the text of the enhanced feed ban statement is lengthy and potentially cumbersome to apply to hard copy or electronic mixing formulae, mixing sheets and other production records, other terms may be acceptable. These include terms such as "prohibited material", "bovine MBM", and "mixed MBM". Abbreviations, symbols or other means can also be used instead of applying the full text of the statement to identify that a product contains PM, provided that:
- the means of identification is explained in written manufacturing procedures;
- it is understood and consistently applied by employees involved in the manufacture of products; and
- records reflect the means described and applied in the manufacture of products containing prohibited material.
Q. Does the enhanced feed ban statement have to appear on invoices for sales of animal food or ingredients containing PM?
A. If the invoice or shipping bill is used as the label (e.g., for bulk feed shipments), then all pertinent information, including the enhanced feed ban statement, must be present on the invoice.
If, however, products are sold in bulk or in packages and the required label, including the enhanced feed ban statement, is provided with the product, the statement is not additionally required on the invoice.
Q. What if an animal food manufacturer switches from not using PM, to using PM, in an animal food for non-ruminants that is registered by the CFIA? Does the registrant have to submit an application for an amendment to the registration?
A. No. For labels of standard (non-specialty) registered products such as complete feeds and supplements, the enhanced feed ban statement is to be applied as outlined in this document. However, the registrant does not have to submit an application for an administrative amendment to the registration, regardless of whether the statement was included or not on the proposed label submitted with the original application.
Scenario 1.1 - No prohibited material contamination prevention measures used
A commercial feed mill receives, stores and handles prohibited material (PM) on a regular basis, and animal foods for ruminants are not manufactured on the premises. The mill does not take any precautions or perform any clean-out procedures to prevent cross-contamination between products not containing PM and products that do contain PM.
Q. Is the enhanced feed ban statement required on all labels and documentation of animal food and other products?
A. Yes. If the mill does not take precautions to prevent cross-contamination or carryover from PM or products containing PM to products not containing PM from occurring at all of the stages of receiving, production, and storage of ingredients and products, the enhanced feed ban statement must appear on the labels of all products produced by the mill.
Q. Does this mean that written procedures are not needed in this case?
A. No. Written procedures documenting that precautionary controls are not employed, and that the enhanced feed ban statement must appear on all labels and documentation, are still required to demonstrate that the risks of cross-contamination are not being managed.
Q. If single ingredient feeds (e.g., cereal grains or oilseed meals) are processed or handled through common equipment in the mill and sold for use in animal food, is the enhanced feed ban statement required on all ingredient product labels and documentation?
A. If precautions are not taken to prevent cross-contamination or carryover of ingredients processed or handled in common equipment by products containing prohibited material, the enhanced feed ban statement must appear on labels of all products, as they contain prohibited material.
Scenario 1.2 - Prohibited material not received, handled or stored
A commercial feed mill does not receive, store or handle prohibited material (PM). However, the feed manufacturer would like to label the animal food manufactured by the mill with the enhanced feed ban statement just in case ingredients or other products coming into the mill may be mislabelled, adulterated or contaminated with PM.
Q. Is the application of the enhanced feed ban statement in this scenario appropriate within the scope of the regulations?
A. No. The enhanced feed ban statement must appear only on labels and documentation of products that contain PM in accordance with the policy principles outlined above. This allows for an accurate representation of the contents of the product to other feed manufacturers, retailers or users of the product.
The use of the enhanced feed ban statement "just in case" on labels and documentation is not a substitute for establishing and employing sound ingredient purchasing, receiving and storage programs for feed manufacturers.
Scenario 1.3 - Prohibited material infrequently received, handled or stored
A commercial feed mill normally makes animal foods for non-ruminant animals that do not contain prohibited material (PM) but, since the mill uses least-cost formulations, it may periodically purchase ingredients or other animal foods that do contain PM.
Q. Can the enhanced feed ban statement be placed on all animal food labels and documentation in this facility?
A. No. If the feed mill is not receiving, storing or handling PM, the enhanced feed ban statement is not to be applied to animal food labels and documentation if they do not contain PM. Whenever ingredients or products that contain PM are purchased for use in the manufacture of animal food, the enhanced feed ban statement must be used within the intent of the regulations. If a feed facility switches between non-prohibited material and PM, flushing or clean out of the complete system would be required.
Scenario 1.4 - Sequencing of production
A commercial feed mill receives, stores and handles prohibited material (PM), and employs a non-dedicated processing line and equipment to manufacture animal foods containing or not containing PM. Depending on the formulation and sequence in which a product is manufactured, the manufacturer wishes to place the enhanced feed ban statement on labels and documentation for only those animal foods that contain PM.
Q. For a lot of animal food manufactured immediately after animal food containing PM, does the statement need to be applied to labels and documentation?
A. Yes. The first lot of animal food manufactured following an animal food containing PM in a processing line or piece of equipment is considered to contain PM, unless the establishment has evidence to demonstrate the contrary.
Scenario 1.5 - Sequencing of storage
A lot of animal food is manufactured without the use of prohibited material (PM), and placed in a bulk bin for storage. A subsequent lot of animal food is then manufactured that contains PM, and is placed on top of the previous lot of animal food in the same bulk bin.
Q. How is the animal food stored in this bin to be labelled?
A. Due to the fact that bulk bins do not allow for separation, any lots containing PM added to and stored in bulk with others that do not, provide no assurance that any of the animal food in storage would not contain PM once unloading and distribution of the animal food begins. Unless separate bins for animal foods containing PM and non-prohibited material are used, animal food labels and documentation for the entire lot of bulk animal food stored in a common bin must carry the enhanced feed ban statement.
Scenario 1.6 - Production line/equipment flushing used as a prohibited material contamination prevention measure
A feed mill receives, stores and handles prohibited material (PM), and manufactures only food for non-ruminant animals. Their written procedures include a requirement to use a flush of the complete feed manufacturing system before animal food not intentionally containing PM is manufactured.
Q. Is animal food produced after the flush required to have the enhanced feed ban statement applied on the label and documentation?
A. The feed mill's written procedures determine whether the conditions of use of a flush following the manufacture of products containing PM are adequate, and which product labels and documentation must carry the enhanced feed ban statement. CFIA inspection staff will verify that flushes are being used in accordance with written procedures and the condition and disposition of the flush material, and will review documentation to confirm that procedures are being followed as written. If the flush is determined to be adequate, the application of the enhanced feed ban statement is not required to appear on the labels and documentation for animal food products that follow a flush.
2. Application of Lot Numbers
Policy Context and Terminology
Lot numbers must be used to uniquely identify every lot of animal food, and recorded so that products can be identified and recalled, if necessary. However, sections 171.(1) and 171.(2) of the Health of Animals Regulations do not specify what form lot numbers must take, or where product information is to be kept. These identifiers can take the form of numbers, but letters, symbols, or combinations of such characters can also be used. The information can be recorded in a separate register that contains product distribution information, maintained in a file of invoices for each lot of animal food distributed, or follow some other approach.
For bulk animal food, a "lot" is a single batch or multiple batches that make up an order, and must be identified by a lot number. For bulk animal food ingredients such as meat and bone meal (MBM), the usual approach is that a day's production is one lot. When product is removed from a silo, the lot number can be the invoice number on the bulk shipment, providing it can be related to the production lot information.
While a lot number is required for every lot of animal food, individual labels or packages in a lot, bills of sale, and invoices are not specifically required to indicate a lot number. However, it must be in the distribution records for the animal food with sufficient detail to enable a product recall, if necessary. Consideration should be given to indicating lot numbers on labels or packages to facilitate lot identification. A date on a tag, label, or bag could be considered a lot number.
Retailers are not obliged to record lot numbers assigned by the manufacturer for animal food that they buy. However, a retailer is obliged to keep records of lot numbers for any animal food products that they sell. The retailer must use a system that allows them to identify the product and the person who purchased it for recall purposes.
3. Retention of Records
Policy Context
Since 1997 there has been a requirement in the Health of Animals Regulations for records to be kept for a two-year period. An amendment to section 171.(1) and 171.(2) which came into force February 1, 2007, in conjunction with an administrative regulatory package (SOR/2007-24), adds a requirement to keep records for 10 years, which replaces the previous two-year requirement. This new requirement applies to every person who manufactures, imports, packages, stores, distributes, sells, or advertises for sale animal food for the species specified in section 171.(1) of the Health of Animals Regulations.
In the event of a change of ownership of a business, obligations regarding the retention of records may still apply after the change has come into effect. Any remaining obligations are dependent on the nature of the business entity (whether the business is a sole proprietorship, a corporation, a co-operative corporation, a partnership, etc.), and the nature of the change (sale, merger, closure, etc.) and other factors. As a result, any obligations that may apply to a specific business will require review and guidance on a case-by-case basis. Please contact a CFIA Feed Program Specialist with the details regarding any change of ownership to obtain guidance regarding obligations that may remain once the change takes effect.
Feed Facility Record Removal and Retention Policy
Questions & Answers
The following questions and answers related to specific scenarios are provided as guidance on the policy principles, their interpretation and application.
Scenario 3.1 - Records for animal food manufactured/ distributed prior to February 1, 2005.
Q. If a CFIA inspector was to inspect an animal food manufacturing or distribution facility today, would the operator be legally obliged to produce records for animal food manufactured or distributed on or before January 31, 2005?
A. No. These records would only have had to be retained for the period established by the 1997 feed ban (two years from the date of the record). Even though the retention period requirement was extended to 10 years as of February 1, 2007, the two-year retention period would have already been satisfied for records dated January 31, 2005 or before.
Scenario 3.2 - Records for animal food manufactured/ distributed between February 1, 2005, and February 1, 2007.
Q. If a CFIA inspector was to inspect an animal food manufacturing or distribution facility today, would the operator be legally obliged to produce records for animal food manufactured or distributed between February 1, 2005, and February 1, 2007?
A. Yes. These records would not have satisfied the retention period requirement established by the 1997 feed ban (two years from the date the animal food was manufactured/distributed), at the time that the requirement was extended to 10 years on February 1, 2007. As a result, the manufacturer or distributor would be obliged to produce records for inspection and retain them for 10 years from the date of the record (for example, records dated February 1, 2006, would have to be retained until February 1, 2016).
Scenario 3.3 - Records for animal food manufactured/ distributed after February 1, 2007.
Q. If a CFIA was to inspect an animal food manufacturing or distribution facility today, would the operator be legally obliged to produce records for animal food manufactured/distributed after February 1, 2007?
A. Yes. Records created after the retention period requirement was extended to 10 years on February 1, 2007, would have to be produced by operators for inspection and be retained for 10 years from the date of the record (for example, records dated February 1, 2008, would have to be retained until February 1, 2018).