General principles
- The Canadian Food Inspection Agency (CFIA) verifies that livestock feeds manufactured and sold in Canada (or imported into Canada) are safe, effective and labelled appropriately
- The Feeds Act and Regulations and the Health of Animals Act and Regulations set out the legal requirements for all feed manufacturers, which includes regulations governing the manufacturing and sale of customer formula feeds
- Customer formula feeds manufactured in Canada may be exempt from registration if regulatory exemption standards are met; all imported mixed feeds must be registered.
- The purpose of this document is to provide clarification to stakeholders, including but not limited to CFIA inspection staff and the Canadian feed industry, on the documentation required to support the manufacturing and labelling of customer formula feeds
Regulatory authority
- A Customer Formula Feed is defined in Section 2 of the Feeds Regulations as:
- a feed that is manufactured by a manufacturer for feeding to his own livestock
- a feed that is manufactured by a manufacturer pursuant to a written order that is signedFootnote 1 by a purchaser and that states the kind and amount of each single ingredient feed to be used in the manufacture of that feed, or
- a feed that is manufactured by a manufacturer pursuant to a written order that is signed by a purchaser and that states the kind and amount of each single ingredient feed to be added to other mixed feeds that would be acceptable for registrationFootnote 2, as a service to the purchaser, and in the case of a feed referred to in paragraph (b) or (c), that is not intended for resale by that purchaser or, where that purchaser is a feed manufacturer, that is not intended for use by that purchaser in the manufacture of a feed referred to in paragraph (b) or (c).
The definition described above provides producers with the flexibility to request the manufacturing of specific feeds based on their knowledge of their livestock's needs, without requiring registration. While the regulations are clear that a signed written order, indicating the name and amount of specific ingredients to be used, must be provided whenever a customer formula feed is to be manufactured, the production mechanics and manufacturing of feeds has evolved since the initial writing of the regulations. Therefore, given the frequency a specific customer formula feed could be ordered by the same customer over a period of time, requiring a signed written order each time is not practical in most cases. Rather, the expectation is that before the customer formula feed is manufactured, the facility will have on-site:
- a signed customer formula that meets the conditions necessary to be exempt from registration (i.e., contains all the required information to conform with one of the options identified in the customer formula definition); and
- a written request that a specific amount of a customer formula feed be manufactured, supplied or furnished. The written request could be submitted by the purchaser or transcribed by the seller and would contain the name and amount of each customer formula product being requested, the date the request was made and the name of the person making and/or transcribing the request.
When these two criteria are met, the full intent of the customer formula feed definition is met.
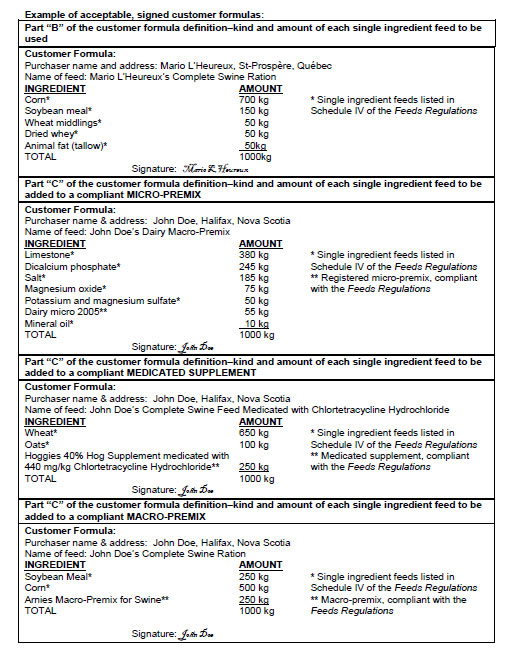

Description for formula - Examples of acceptable, signed customer formulas
Part "B" of the customer formula definition–kind and amount of each single ingredient feed to be used
Customer Formula:
Purchaser name and address - example: Jane Doe, Red Deer, Alberta
Name of feed - example: Jane Doe's Complete Swine Ration
Ingredients and amounts – example: Corn, 700 kilograms
Soybean meal, 150 kilograms
Wheat middlings, 50 kilograms
Dried whey, 50 kilograms
Animal fat (tallow), 50 kilograms
Total amount – example: 1000 kilograms
Signature – example: Jane Doe
Note that single ingredient feeds are listed in Schedule IV of the Feeds Regulations
Part "C" of the customer formula definition–kind and amount of each single ingredient feed to be added to a compliant micro-premix
Customer Formula:
Purchaser name and address - example: John Doe, Halifax, Nova Scotia
Name of feed - example: John Doe's Dairy Macro-Premix
Ingredients and amounts – example: Limestone, 380 kilograms
Dicalcium phosphate, 245 kilograms
Salt, 185 kilograms
Magnesium oxide, 75 kilograms
Potassium and magnesium sulfate, 50 kilograms
Dairy micro 2005, 55 kilograms
Mineral oil, 10 kilograms
Total amount – example: 1000 kilograms
Signature – example: John Doe
Note that single ingredient feeds are listed in Schedule IV of the Feeds Regulations and Dairy micro 2005 is a registered micro-premix compliant with the Feeds Regulations
Part "C" of the customer formula definition–kind and amount of each single ingredient feed to be added to a compliant medicated supplement
Customer Formula:
Purchaser name and address - example: John Doe, Halifax, Nova Scotia
Name of feed - example: John Doe's Complete Swine Feed Medicated with Chlortetracycline Hydrochloride
Ingredients and amounts – example: Wheat, 650 kilograms
Oats, 100 kilograms
Hoggies 40% Hog Supplement medicated with 440 mg/kg , 250 kilograms
Total amount – example: 1000 kg
Signature – example: John Doe
Note that single ingredient feeds are listed in Schedule IV of the Feeds Regulations and Hoggies 40% Hog Supplement medicated with 440 mg/kg is a medicated supplement compliant with the Feeds Regulations
Part "C" of the customer formula definition–kind and amount of each single ingredient feed to be added to a compliant macro-premix
Customer Formula:
Purchaser name and address - example: John Doe, Halifax, Nova Scotia
Name of feed - example: John Doe's Complete Swine Ration
Ingredients and amounts – example: Soybean Meal, 250 kilograms
Corn, 500 kilograms
Arnies Macro-Premix for Swine, 250 kilograms
Total amount – example: 1000 kg
Signature – example: John Doe
Note that single ingredient feeds are listed in Schedule IV of the Feeds Regulations and Arnies Macro-Premix for Swine is a macro-premix compliant with the Feeds Regulations
Part "C" of the customer formula definition–kind and amount of each single ingredient feed to be added to a compliant medicated complete feed
Customer Formula:
Purchaser name and address - example: John Doe, Halifax, Nova Scotia
Name of feed – example: John Doe's Complete Swine Starter Feed Medicated with Virginiamycin
Ingredients and amounts – example: Anybrand pig starter medicated with Virginiamycin, 998 kilograms
Zinc Oxide Anhydrous, 2 kilograms
Total amount – example: 1000 kilograms
Signature – example: Joe Doe
Note that single ingredient feeds are listed in Schedule IV of the Feeds Regulations and Anybrand pig starter medicated with Virginiamycin is a medicated complete feed compliant with the Feeds Regulations
- The examples above show the addition of single ingredient feeds to a mixed feed (premix/supplement or complete feed) it is permissible for the mixing formula/mixing sheet for these customer formula feeds to include all the ingredients within the respective mixed feed, in addition to the single ingredients listed in the customer formula.
- A document signed by a purchaser that lists the guaranteed analysis of a particular feed rather than the actual single ingredients and/or mixed feeds to be used, does not meet the regulatory definition of a customer formula feed and thus would be found in contravention of the Feeds Regulations.
- Manufacturers that do not meet the exemption from registration criteria outlined in the customer formula feed definitions may consider applying for registration of these products.
- A signed customer formula that meets the conditions necessary to be exempt from registration may be faxed or emailed to the commercial facility for manufacturing purposes as long as the original signed formula is in the manufacturer's possession prior to distribution of the customer formula feed
Regulatory requirements for customer formula feeds
Exemption from registration
- must be manufactured in Canada
- a signed customer formula that meets the following standards is in possession of the manufacturer prior to the manufacture of the feed
- identifies only approved single ingredient feeds or mixed feeds in compliance with the regulations
- does not identify in-house micro premixesFootnote 3 or DIN productsFootnote 4 as they are neither single ingredient feeds nor mixed feeds acceptable for registration
- does not identify or include other customer formula feeds
- a signed written order must be in possession of the manufacturer prior to the manufacture of the feed (one signed written order for each customer formula feed to be manufactured) or
- a signed customer formula is in possession of the manufacturer and customer formula feeds are manufactured only after the signed customer formula and written request are received specifying the amount of the specific customer formula feed to be manufactured
- must not be resold
- intermediate sales (e.g. manufacturer to retailer to supplier of formula) are permitted as long as the final purchaser is the person that supplied the customer formula, the label contains the required information at all times and a copy of the signed customer formula and written request is in the possession of the manufacturer and the other individuals handling the customer formula feed
- must not be used to make different customer formula feeds by the manufacturer
- medicated customer formula feeds must meet part "c" of the customer formula definition where single ingredients are added to a medicated mixed feed acceptable for registration
- the unique name of the mixed feed (including the medication for medicated CMIB feeds) or a product code or other form of identification that will permit the assessment of compliance of the mixed feed used (medicated or not) must be listed on the signed customer formula
- copies of signed customer formula and a list of each date the feed was manufactured must be kept for at least six months from the last date of manufacture of that feed
Floor stocking
- not permitted to floor stock customer formula feeds
- CFIA inspectors are asked to confirm the facility has in its possession a signed customer formula and that customer formula feeds are produced only after a request is received from the purchaser indicating the amount of feed to manufacture. The practice of manufacturing customer formula feeds prior to receiving a request and floor stocking those feeds in anticipation of future orders would be deemed contrary to the regulatory definition and thus a contravention of the Feeds Regulations
Toll manufacturing
- an arrangement whereby a first firm with specialized equipment processes raw materials or semi-finished goods for a second firm, e.g. Feed Mill #1 requests Feed Mill #2 to manufacture a customer formula feed on their behalf due to the fact that Feed Mill #2 has specialized equipment
- toll manufacturing is permitted when copies of the signed customer formula and written request is provided to the toll manufacturer and the product is labelled as prescribed at the time of manufacturing which includes the name and address of the toll manufacturer as well as the name and address of the customer supplying the formula
Veterinary prescriptions and customer formula feeds
- where a veterinarian prescribes the addition of a medication to a customer formula feed, the resulting mixture is considered a veterinary prescription feed and must meet the requirements outlined in section 5(2)(g) (exemption from registration for a veterinary prescription feed) and section 26(7) (labelling) of the Feeds Regulations
- the medicating ingredient(s) indicated on the veterinarian prescription must be added to the specific customer formula feed identified by the veterinarian on the prescription
- the customer formula feed must exist initially and fully comply with the applicable standards outlined in the Feeds Regulations. The medicating ingredient(s) from the veterinary prescription are then added to that customer formula feed
- in addition to the labelling requirements prescribed in subsection 26(1), the veterinary prescription feed label must include the requirements specified in section 26(7)(a)to(g) of the Feeds Regulations (additional labelling requirements for veterinary prescription feeds)
- in the case of a veterinary prescription feed, the mixing formula/mixing sheet can show the addition of a drug premix (DIN product) to the customer formula feed to supply medications outside the approved levels identified in the CMIBFootnote 5
Optional labelling requirements for customer formula feeds
- Recognized nutrient guarantees (i.e. those listed in Table 3 of the Feeds Regulations) are permitted and must accurately reflect the composition of the customer formula feed
- A complete list of ingredients is permitted as long as all ingredients are listed and are those identified on the signed customer formula
- Directions for use are permitted provided that they are not misleading
- The selenium supplementation table for lactating and dry dairy cattle is permitted
Mandatory labelling informationFootnote 6 for customer formula feeds
Non-medicated customer formula feeds
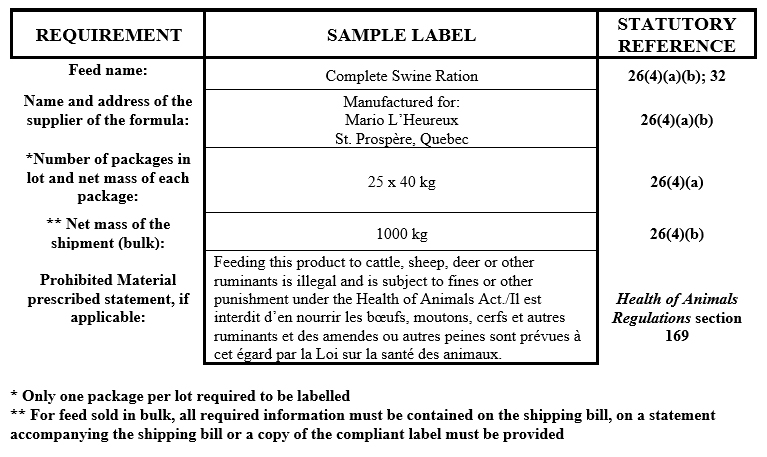
Description for formula - Non-medicated customer formula feeds
- feed name as per Section 32 of the Feeds Regulations
- the name and address of the supplier of the formula
- if in bulk, the net mass of the shipment
- if in bags, only one package per lot requires a label and must indicate: the net mass of each individual package and the number of packages in the lot
- if feed contains prohibited material, the prescribed statement as defined in the Health of Animals Regulations
Medicated customer formula feeds
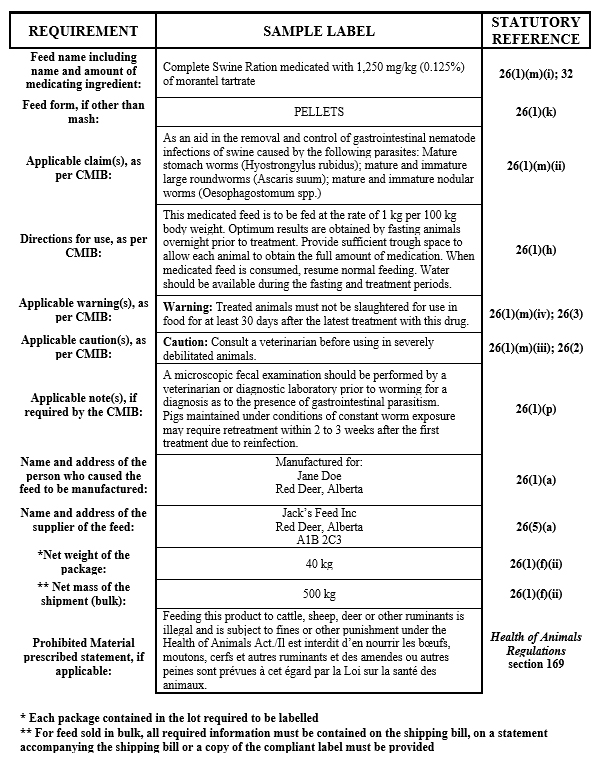
Description for formula - Medicated customer formula feeds
- feed name as per Section 32 of the Feeds Regulations
- the name and address of the supplier of the feed
- name and address of the person who caused the feed to be manufactured
- net amount of feed
- form of the feed, if other than mash
- ID code if a Micro Premix
- name and amount of medicating ingredient, in direct association with the feed name
- directions for use
- appropriate CMIB claim(s)
- required CMIB caution(s) and/or warning(s)
- if in bags, each bag must be labelled
- if in bulk, a copy of the compliant label must be provided to the purchaser
- if feed contains prohibited material, the prescribed statement as defined in the Health of Animals Regulations
Veterinary prescription customer formula feeds
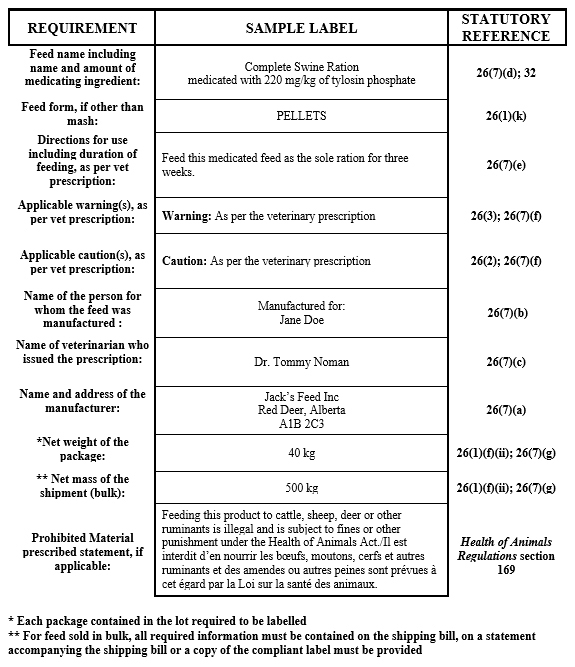
Description for formula - Veterinary prescription customer formula feeds
- name of the feed including the name and amount of medicating ingredient present in the feed, as per the veterinary prescription
- name of the person for whom the feed was manufactured
- name and address of the manufacturer
- net amount
- form of the feed, if other than mash
- directions for use including duration of feeding, as per the veterinary prescription
- name of veterinarian who issued the prescription
- any required caution(s) and/or warning(s) as indicated on the veterinary prescription
- if in bags, each bag must be labelled
- if in bulk, a copy of the compliant label must be provided to the purchaser
- if feed contains prohibited material, the prescribed statement as defined in the Health of Animals Regulations
Questions regarding this policy should be forwarded to:
Phil Snelgrove
National Manager
Feed Inspection Support &Training Section
Animal Feed Division
Canadian Food Inspection Agency
59 Camelot Drive
Ottawa, Ontario K1A 0Y9
Telephone: 613-773-7514
Facsimile: 613-773-7565
Email: philip.snelgrove@inspection.gc.ca
References:
- Feeds Act and Regulations
- Health of Animals Act and Regulations
- RG-1 Regulatory Guidance: Feed Registration Procedures and Labelling Standards Chapter 3 – Specific Registration Information by Feed Type – 3.12 Micro Premixes
- RG-1 Regulatory Guidance: Feed Registration Procedures and Labelling Standards Chapter 4 – Labeling and Guarantees – 4.1 Labelling of Livestock Feeds