Feed Ingredient Assessment and Authorization – Modernized Regulatory Framework Proposal – Consultation Summary – Respondent Comments and CFIA Responses
Introduction
This page has been archived
Information identified as archived is provided for reference, research or record-keeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
The Canadian Food Inspection Agency (CFIA) has embarked on a comprehensive change agenda to strengthen its foundation of legislation, regulatory programs and inspection delivery. These directions set the context for the renewal of the Feeds Regulations (Regulations).
The goal of modernizing the Regulations is to reduce compliance burden and support innovation while maintaining animal and human health, as well as environmental and economic stability. The modernization of the Regulations is being designed to benefit the collective Canadian feed industry which includes commercial feed manufacturers, retailers, importers, exporters, ingredient manufacturers, and farmers. As well as aligning with other international feed regulatory regimes, modernization also maintains the objective of enhancing animal health and food safety for the Canadian public.
Within the scope of the current feed regulatory framework, all feed ingredients require pre-market assessment and authorization prior to manufacture, sale and importation for feeding to livestock. Ingredients are assessed for efficacy and safety in the interest of protecting human health (food safety and occupational health), animal health and the environment. Once authorized, feed ingredients are listed in Schedules IV or V of the Regulations. Safe, effective feeds and feed ingredients contribute to the production of safe food products as well as healthy and efficient livestock.
About the Consultation
Following preliminary pre-consultation activities with stakeholders and interested parties during 2012, the Agency embarked on further consultation in 2013 by way of more subject-specific workshops and proposals (or "modules") given the comprehensive, complex nature of the feed regulatory modernization project. To this end, the Agency prepared and posted the module, Feed Ingredient Assessment and Authorization - Regulatory Framework Proposal, and undertook a 30-day consultation on feed ingredient regulation to:
- map out a six-step authorization process (Figure 1); and
- propose changes to current processes and requirements to be applied in a modernized ingredient assessment and authorization framework.
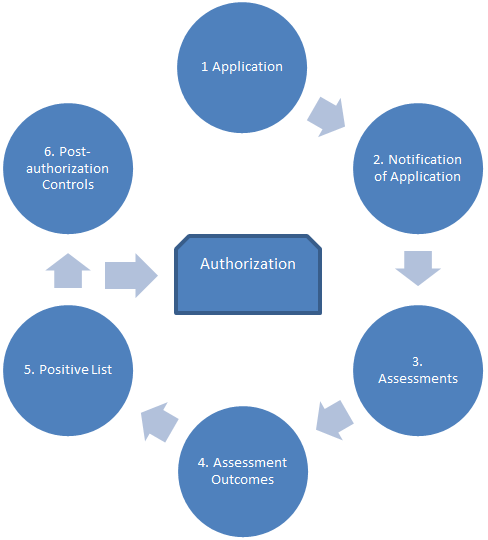
Description of Figure 1
The figure illustrates, by way of labelled bubbles organized in a circle and joined to each other with arrows, a proposed series of six steps that the CFIA will follow in the assessment and authorization of feed ingredients:
- Application;
- Notification of Application;
- Assessments;
- Assessment Outcomes;
- Positive List; and
- Post-authorization Controls.
In the centre of this circle of six bubbles is a rectangle labelled as Authorization; it is linked to the bubbles by an arrow between steps 5 and 6 to indicate that an ingredient would be authorized in this proposed process following the Positive List step.
The CFIA invited comments from interested parties from November 20, 2013 to December 20, 2013.
The primary mechanisms to facilitate the consultation involved the:
- direct distribution of the proposal to stakeholder groups, individuals and other interested parties who have been engaged in Agency feed regulatory modernization consultation activities to date;
- posting of the feed ingredient proposal on the CFIA website; and
- outreach directly to industry stakeholders by Agency staff.
Twenty-one (21) responses were received in respect of this consultation and two (2) additional responses received during pre-consultation in 2012 specifically regarding feed ingredient assessment and authorization have been considered in the preparation of this summary.
This report consolidates and summarizes the comments received during this consultation and the pre-consultation period that directly pertain to the feed ingredient proposal and the CFIA's response to those comments.
- Date modified: