Table of Contents
- 1 Introduction
- 2 Creation of a quarantine site diagram
- 3 Creation of standard operating procedures
- Appendix A: Example quarantine site diagram
- Appendix B: Example standard operating procedure for quarantine
- Appendix C: Standard operating procedure guidelines for import quarantine
- Appendix D: Example quarantine access log
- Appendix E: Susceptible Species
1 Introduction
The purpose of this document is to provide importers with guidance when establishing a quarantine unit for approval by the Canadian Food Inspection Agency (CFIA) for international imports.
Key elements for quarantine unit creation include the following:
- if possible, a hydrogeological survey of groundwater supplies;
- a site diagram indicating the location of the physical infrastructure on the premises, the water flow patterns for the site and the locations of the different populations of animals on the site, if multiple populations are present;
- written standard of procedures and written records that demonstrate compliance with the operating procedures; and
- written records that demonstrate the implementation of active observational surveillance and traceability.
The importer should have quarantine unit staff that are trained and knowledgeable in the areas of biosecurity and aquatic animal diseases, or have a working relationship with an aquatic animal health professional or veterinarian.
This document is not meant to be a comprehensive list of requirements and is to be used as guidance for the establishment of a quarantine unit. The specific requirements of a particular quarantine unit are dependent on the individual premises, location of the quarantine unit, and life stage/species/product being imported.
1.1 Definitions
- 100-year flood
- is the peak or flood flow with one chance in one hundred of occurring in any given year. (Crue centenaire)
- Access points
- initial control points with defined entry and exit procedures. (Points d'accès)
- Active Observational Surveillance
- the process of actively and systematically looking for diseased animals by a trained individual on a frequent, pre-planned, and ongoing basis, where a predefined plan of action is implemented when affected animals are discovered. (Surveillance par observation active)
- Ante-room
- is a predefined area that separates the quarantine unit from the surrounding environment. It may not be a room, but rather a designated area on the site where exterior clothing is exchanged for site specific clothing and a hand wash station is provided. (Antichambre)
- Aquatic animal
- means any finfish, mollusc or crustacean, or any part of a finfish, mollusc or crustacean at any life stage, as well as any germplasm of those animals. (Health of Animals, Part XVI, Section 190) (Animal aquatique)
- Biological vector
- is an organism, usually an arthropod, in whose body the pathogen develops or multiplies before becoming infective for the recipient individual. (Vecteur biologique)
- Competent Authority
- means the Veterinary Authority or other Governmental Authority of a Member having the responsibility and competence for ensuring or supervising the implementation of aquatic animal health and welfare measures, international health certification and other standards and recommendations in the Aquatic Code in the whole territory. (Aquatic Animal Health Code 2012)(Autorité compétente)
- Effluent water
- is the waste water from an aquatic animal tank or holding system. Refer to "Solid waste." (Effluents)
- Equipment
- refers to all inanimate objects that can be used in the quarantine unit, including but not limited to equipment for aquatic animal handling, feeding, mortality collection and disposal as well as person protective equipment such as boots and raingear, graders, and fish pumps. (Équipement)
- Fomite
- Any inanimate object or substance capable of carrying infectious organisms. This may include but is not limited to equipment, vehicles and articles of clothing or shoes. (Vecteur passif)
- Germplasm
- means semen, male or female germ cells or genetic material taken from a male or female germ cell for the purpose of producing a zygote and includes embryos but does not include a hatching egg. (Health of Animals Regulations) (Matériel génétique)
- Import
- means entry into Canada from any other origin. (Importation)
- Influent water
- is the inflow of water into an aquatic animal tank or holding system. (Eau d'arrivée)
- Inorganic waste
- is material that is non-decomposable and does not consist of animal and/or plant matter. (Déchets inorganiques)
- Inspection
- means an inspection carried out by an inspector, except where a customs officer carries out an inspection pursuant to section 16 of the Health of Animals Act. (Health of Animals Regulations) (Inspection)
- Inspector
- means a person who is designated as an inspector, pursuant to subsection 13(3) of the Canadian Food Inspection Agency Act, for the purposes of section 32 of the Health of Animals Act and/or subsection 17 of the Fish Inspection Act. (Inspecteur)
- Mechanical vector
- is a living organism that can passively transmit the pathogen to an aquatic animal but is not infected by the pathogen (and therefore cannot actively shed the pathogen). (Vecteur mécanique)
- Organic waste
- is material that is decomposable and consists of animal and/or plant matter. (Déchets organiques)
- Pathogens of concern
- refers to the infectious agents in which the aquatic animals are considered to be susceptible. (Agents pathogènes préoccupants)
- Permit
- refers to an aquatic animal health import permit issued pursuant to Section 160 of the Health of Animals Act. (Permis)
- Population
- means a group of aquatic animals that are linked by direct or indirect contact with each other. The number of populations in a quarantine unit is determined by the links between the animals within the quarantine unit and the risk factors (animals, water, feed, fomites and vectors) that influence the health status of the quarantine unit. (Population)
- Reportable
- means prescribed as reportable by the minister as listed in the Reportable Diseases Regulations. (À déclaration obligatoire)
- Risk assessment
- means the evaluation of the likelihood and the biological and economic consequences of entry, establishment and spread of a hazard within the territory of an importing country. (Aquatic Animal Health Code 2013) (Évaluation des risques)
- Sample
- means a defined number of aquatic animals taken from a population of aquatic animals for the purpose of testing or collection of specimens. (Échantillon)
- Solid waste
- is inorganic or organic material that is present in transportation, influent, and effluent water. (Déchets solides)
- Specimen
- means a tissue, organ or bodily fluid removed from an aquatic animal for the purpose of testing. (Spécimen)
- Veterinary inspector
- means a veterinarian designated as an inspector pursuant to section 32 of the Health of Animals Act. (Vétérinaire-inspecteur)
2 Creation of a quarantine site diagram
Quarantine site diagrams are required for all quarantine units unless otherwise stated by the CFIA. Site diagrams can be hand-drawn or computer-generated. It is recommended that a base diagram of the physical infrastructure is first created upon which the other elements can be superimposed (e.g. water flow, animal population, and traffic flow patterns) as it is recognized that these elements may change. Refer to Appendix A: Quarantine Site Diagram for an example diagram of each of the following three steps listed below.
- Add basic physical infrastructure
Main components in a quarantine site diagram:
- Quarantine unit or building encompassing the quarantine unit
- Separate rooms in the quarantine unit (e.g. office, laboratory, and feed storage)
- Water supply
- Infrastructure (e.g. location of aeration towers, water treatment, shipping and receiving areas, and parking)
- Aquatic animal holding area
- Effluent water (e.g. settling ponds, septic tanks, and discharge location)
- Water flow pattern
- Restrictions of movement of people, animals, or vehicle traffic (e.g. gates and fences
- Superimpose water flow pattern
- Add aquatic animal locations
3 Creation of standard operating procedures
Appendix C: Standard operating procedure guidelines for import quarantine consists of a list of standard operating procedures (SOP) and their guidelines for a quarantine unit. The importer is to identify SOPs applicable to their particular quarantine unit from the list in Appendix C and additional procedures and more information may be required under certain circumstances as determined by the CFIA. Not all of the SOPs listed necessarily apply to every quarantine unit, and in some cases SOPs can be combined.
3.1 How to write a SOP
A SOP provides a short set of instructions sufficient to tell staff members how to conduct the procedure. It is primarily an instructional document. Detailed reference material to support the SOP can be appended if desired, but is not necessary; the document's primary function is to instruct staff in simple terms how to carry out the required procedure.
A SOP contains several usual elements that tell the user the following:
- who is responsible for conducting the procedure;
- what to do;
- how to do it;
- required equipment or supplies and where they are located;
- frequency to conduct the procedure; and
- the critical parameters/occurrences that require immediate action or notification of management.
Refer to Appendix B: Example Standard Operating Procedure (SOP) for Quarantine for an example SOP.
3.1.1 SOP elements
The following headings may be included in each written procedure, although not all are necessary every time. Importers may wish to add additional sections or information for their own purposes.
- Title:
- Provide a descriptive title.
- Rationale:
- Explain why the procedure is being conducted.
- Definitions:
- List any definitions or acronyms required to understand the SOP.
- Responsibility:
- Define who is responsible for the information in the SOP and who is responsible for conducting it. Use position titles or people's names to indicate responsibility.
- Equipment and supplies:
- List all equipment required (if any) to complete the SOP.
- Safety concerns:
- List any worker safety or animal health concerns that must be addressed.
- Procedure:
- Describe, in active voice, each step required to complete the SOP, the frequency with which it is conducted, and any critical findings/occurrences that require notification of management or implementation of a contingency plan.
- Contingency plan:
- Outline the actions to be taken should a critical finding occur (e.g. an influent screen found to be damaged must be replaced or repaired as soon as possible).
- Record keeping:
- List all records required, where and how they are stored, and how long they must be maintained. Provide an example of how to fill out records, if desired.
3.1.1.1 Record-keeping requirements
Records are kept to demonstrate that the required quarantine unit procedures are conducted. Record keeping is variable for each activity.
3.2 Guidance applicable to the creation of a quarantine unit
3.2.1 Animals
3.2.1.1 Unintentional Animal Introductions into the Quarantine Unit
Influent and effluent screens are necessary to prevent inadvertent animal introductions; however, there are circumstances where they may not be required (e.g. influent screens are not required if water source is municipal water or ground water; and effluent screens are not required if water is treated and there is a physical barrier preventing invasion of all aquatic animals into the quarantine unit through the effluent system). Screen size is recommended to be no larger than the minimum size of aquatic animal that is possibly present in the influent and effluent water source. Choice of screen size will be a consideration when quarantine units request the acceptance of screening as a physical barrier to prevent invasion of other aquatic animals.
Influent and effluent screens must undergo routine inspections and maintenance and/or repairs as necessary to prevent the unintentional introduction of aquatic animals into the quarantine unit. Influent and effluent screens must be cleaned and maintained on a regular basis to ensure their effectiveness. The screens must be clear of debris and must not exhibit damage.
3.2.1.2 Intentional Animal Introductions into the Quarantine Unit
All aquatic animal introductions must be in compliance with import permit conditions and only the aquatic animals listed on the import permit can be introduced into the quarantine unit. A single quarantine unit must contain only the aquatic animals to be imported as specified on the import permit and a single quarantine unit can contain more than one population with the written preapproval of the CFIA. If multiple populations are introduced into a quarantine unit during the quarantine period, they are considered to have the same health status. The quarantine unit must demonstrate traceability so that in the event of a disease outbreak, trace-in and trace-out can be completed.
3.2.1.3 Aquatic Animal Gametes and/or Germplasm Treatment
Aquatic animal gametes and/or germplasm must be treated prior to export in the foreign country or upon arrival into the quarantine unit. The products used for treatment must be effective against the pathogens of concern specific to the species being imported, compliant with international standards, used in accordance with manufacturer's recommendations, and approved by the CFIA.
Importers are strongly encouraged to follow the World Organisation for Animal Health (WOAH; founded as Office International des Épizooties (OIE)) recommendations for surface disinfection of salmonid eggs in the WOAH Aquatic Animal Health Code, 24th Edition, 2021, Chapter 4.4.
3.2.2 Water
3.2.2.1 Unintentional Introduction of Water into the Quarantine Unit
The quarantine unit should be located in an area that is outside the area to be affected by a 100-year flood. If the quarantine unit resides within a 100-year flood area, sufficient dikes/levees must surround it to prevent flooding or other mitigation measures must be implemented.
A 100-year flood is the peak or flood flow with one chance in one hundred of occurring in any given year. Refer to Environment Canada and provincial governments for identification of flood areas and risks applicable to the region of the quarantine unit.
3.2.2.2 Treatment and Disposal of Water and/or Ice Accompanying Imported Aquatic Animals for Quarantine
Whenever possible, transportation water should be disinfected within transportation containers after the transfer of aquatic animals into the quarantine unit. If applicable, transportation ice must be melted prior to treatment. Low levels of suspended solid waste present in the transportation containers can be disinfected and disposed of with the transportation water. Significant amount of solid waste present in the transportation containers must be settled or filtered out of the water in order for the water treatment to be efficacious. Solid waste must either be stored in a leak-proof holding container within the quarantine unit until the aquatic animals are released from quarantine or treated with an approved method to destroy the pathogens of concern prior to disposal.
The method selected to treat transportation water must have supporting evidence, such as scientific literature, demonstrating it as efficacious against pathogens of concern. The transportation water can be introduced into the quarantine unit if the effluent treatment system in the quarantine unit can sufficiently handle the volume of transportation water and effectively treat the pathogens of concern. In order to determine if the effluent treatment system can handle the volume of transportation water, the importer must assess the effluent treatment system in the quarantine unit and provide evidence of its capacity and capabilities.
3.2.2.3 Assessment of Influent Water Source
Water sources that are pathogen free and acceptable as influent water sources for a quarantine unit include ground water, spring water, de-chlorinated municipal water supplies, and marine premises that use manufactured sea salts (such as Instant Ocean) mixed with pathogen free freshwater. Other sources of water such as surface water would need to be screened, filtered, and treated by a method demonstrated to be effective for elimination of pathogens of concern to be acceptable for use.
All influent water sources for the quarantine unit would be subject to an assessment prior to approval of the quarantine unit. For ground or spring water sources, information needed for the assessment could include records pertaining to the well depth (if applicable), land use for surrounding area (and local industries), water source classification (confined aquifer or unconfined aquifer), the history of the water quality, the changes in water temperature over the course of one year and other water-quality parameters, the soil or rock type into which the well is drilled, dug or bored, and any data available for the testing of the water for the presence of pathogens of concern.
The likelihood of groundwater being contaminated by surface water supplies depends on the soil/rock types in which the wells have been dug, drilled or bored; the depth of the well; and the distance from surface water supplies. The make-up of the confining bed over the ground water supply influences the ability of surface water to penetrate the substrate and reach ground water. Where the confining bed is impermeable, creating a confined aquifer, it is improbable that contaminated surface water has reached the water in the confined aquifer. Where the aquifer is unconfined, that is the confining bed is permeable, it is more likely that the ground water supply can be contaminated by surface waters. Water temperature changes indicate the proximity of the water to the surface and deep wells will show virtually no temperature fluctuation. Shallower wells will have greater temperature fluctuation (up to 5°C to 10°C).
Reference:
Heath, RC. Basic Ground Water Hydrology. US Geological Service Water Supply Paper 1983:2220.
3.2.2.4 Water Quality Monitoring
The maintenance of water quality is critical to the quarantine unit and specific water quality parameters can impact the water treatment method chosen and the efficacy of water treatment, equipment disinfection, and egg disinfection procedures. At a minimum, water temperature, pH, and turbidity must be monitored and maintained within a range that is acceptable according to the treatment system in the quarantine unit. Water quality monitoring, including the parameters to be measured and the frequency of measurement, is specific to the quarantine unit and will vary with the water source and type of system (e.g. recirculation versus flow through).
Water quality should be monitored consistently and accurately, in order to be confident in the water quality parameters that impact water treatment, egg disinfection and equipment disinfection activities. Acceptable parameters vary depending on the disinfection methods used for water treatment, egg disinfection or equipment disinfection. The importer should take into account the natural, seasonal water quality changes. Water quality should be measured frequently enough to differentiate normal variation from declining water quality conditions. Acceptable water quality parameters and the expected actions of staff if they encounter an unacceptable result should be predetermined.
Water quality parameters that may impact the efficacy of influent, effluent and transportation water treatment are as follows:
- pH;
- water temperature;
- turbidity;
- total dissolved solids;
- total suspended solids;
- iron manganese;
- hydrogen sulphide;
- calcium magnesium;
- residual ozone levels; and
- any other parameters that need to be measured to ensure disinfection systems are effective.
3.2.2.5 Influent and Effluent Water Treatment
Influent and effluent water treatment must ensure log 4 reduction of the pathogens of concern.
Acceptable influent water sources include municipal water that is treated to drinking water standards and ground water that is proven to not be influenced by surface water. Other sources of influent water are acceptable when pre-filtered and treated using one of the following methods that is effective to destroy the pathogens of concern to which the aquatic animals are susceptible: chlorination/de-chlorination, ultraviolet light, ozone, autoclave, electrolytic water treatment, and any other method that is demonstrated to be sufficient to destroy the pathogens of concern and is subsequently approved by the CFIA.
Effluent water must be pre-filtered and treated using one of the following methods that is effective to destroy the pathogens of concern to which the aquatic animals are susceptible: chlorination/de-chlorination, ultraviolet light, ozone, autoclave, electrolytic water treatment, and any other method that is demonstrated to be sufficient to destroy the pathogens of concern and is subsequently approved by the CFIA.
Settling and filtration
Influent water sources other than municipal and ground water must be pre-filtered prior to treatment. Effluent water must always be pre-filtered prior to treatment.
The selection of settling and filtration systems is dependent on the characteristics (type, size, and density) of the particles suspended in the water supply and the flow rate of the system.
Efficacy of water settling and filtration prior to disinfection methods are determined via:
- evaluation of manufacturer's specifications;
- documentation of routine required maintenance;
- monitoring of turbidity or organic loading of the systems prior to the chosen water treatment method; and
- specific pre-treatment requirements of each disinfection system.
Chlorination/de-chlorination
- The level of organic matter and nitrogenous wastes in the water affect the amount of chlorine that must be added to the system; these compounds affect the ability of the chlorine molecules to disinfect. Organic matter and nitrogenous wastes must be measured and removed from the water prior to chlorination.
- Monitoring of the free chlorine levels in the system is done at least daily, both for efficacy and for prevention of toxicity to aquatic animals in the rearing system.
Ultraviolet disinfection
- Sufficient sizing of the UV system: there must be sufficient UV C output performance (typically requiring low pressure bulbs), including accounting for 40% loss of bulb strength over the bulb lifespan. UV C is a subtype of Ultraviolet light.
- Transmissibility of the water to be disinfected must be known (90-95% transmittance required); therefore, pre-filtration of most water supplies is required.
- The operating water flow rate must be known in order to calculate the actual dosage being delivered.
- Dosage and exposure level, thus the sizing of the system, must be targeted to the pathogen(s) of concern.
- The frequency of UV bulb changes must follow manufacturer's specifications.
- Quartz sleeve maintenance requirements depend on the water quality, thus the probability of scale and biofilm deposits on the sleeve. Maintenance schedules must follow manufacturer's recommendations.
- All water to be treated must pass through a filter capable of removing suspended organic material prior to irradiation; this can include use of sand, drum, bead, or other physical filters, reverse osmosis and/or ozone treatment of the water to increase particle size for ease of removal.
- Specifications of the individual systems with measurement of the water transmittance is used as the determination of whether a system is acceptable for the specific situation.
Disinfection using Ozonation
- The ozone demand of the treatment system is dependent on flow rate and water quality. The ozone demands must be known by the quarantine unit staff as pre-filtration and ozone concentration/exposure is varied depending on the system demands.
- Ozone levels must be monitored to verify successful levels achieved.
- Residual ozone must be removed prior to contact with aquatic animal populations.
Electrolysis
- Currently, electrolysis is not used extensively.
- NaCl must be present in the system.
- Measurement of residual hypochlorite and hypobromite is required to determine ongoing efficacy.
Other methods
- Other methods can be considered and require approval by the CFIA.
The importer must demonstrate proper treatment system maintenance for the chosen quarantine unit treatment method.
3.2.2.6 Disposal of Solid Waste Collected from the Quarantine Unit
Solid waste is inorganic or organic material that is present in transportation, influent, and effluent water.
Solid waste must be stored in secure containers within the quarantine unit until it is treated to destroy the pathogens of concern. Waste may be disposed of during the quarantine period upon approval by CFIA inspection when treated prior to disposal; the treatment system must be sufficient to destroy the pathogens of concern to which the aquatic animals are susceptible for the quarantine unit. Several treatment systems are acceptable for quarantine, including rendering, incineration, sterilization, or another method approved by the CFIA. The quarantine unit must either have disposal to municipal sewer or septic system after treatment or disposal to landfill after treatment. The quarantine unit must comply with all provincial and municipal waste management regulations.
3.2.3 Feed
3.2.3.1 Feed Types and Sources
The following feed types are acceptable:
- Live and/or raw unprocessed feed sources from species not susceptible to the pathogens of concern for the species that the feed is being fed to;
- Live and/or unprocessed feed sources that have been certified by the competent authority at the origin of the feed source as free for the pathogens of concern for the species that the feed is being fed to; and
- Dried, pelleted feed sources that have undergone a manufacturing process that is sufficient to destroy the pathogens of concern for the species that the feed is being fed to. Dry, commercially produced feed where total moisture content is less than 10% are negligible risk feed sources.
All feed must be from sources or manufacturers that have methods in place to protect against cross contamination with other feeds, aquatic animals or aquatic animal products between source and delivery at the quarantine unit. Any aquatic animal feed source that is not one of the acceptable feed sources listed above being used in the quarantine unit must undergo a risk assessment by the CFIA prior to its approval for use.
Dry Pelleted, Extruded Feed and Flake Feeds
In general:
- Dry pelleted and extruded feeds generally represent a negligible risk of pathogen introduction due to the temperatures reached during the manufacturing processes for the production of fish meal (75°C to 100°C) and the extrusion/pelletizing process itself; temperatures reached during extrusion can range from 90°C to >120°C which is sufficient to kill all pathogens of concern.
- Flake feeds are highly processed, cooked and dried, and thus represent a negligible risk of pathogen introduction.
Moist or Semi-Moist Feeds
- Due to the moisture content of moist and semi-moist feeds and the lower temperatures of processing, the risk of pathogen transfer in these feeds is elevated over that of dry or extruded pellets.
- The manufacturing process for moist feeds is typically a cold process, and thus the aquatic animal carcasses/offal used for manufacturing must come from fish sources that are determined by the CFIA to represent a negligible risk of pathogen introduction if these feeds are used.
Live Feeds
- If live aquatic animals are to be used as feed for aquatic animals within a quarantine unit, the live feed must be of negligible risk for the pathogens of concern OR if considered susceptible to the same diseases of concern as the aquatic animals in the quarantine unit, the live feed must be certified as free of diseases of concern prior to use as feed.
- Once live animals to be used as aquatic animal feed are introduced into a quarantine unit, they are considered to have the same health status as the aquatic animal population in the quarantine unit.
Offal/Aquatic Animal Waste/Lyophilization
- Raw offal or aquatic animal waste used as aquatic animal feed in the quarantine unit requires risk assessment by the CFIA to determine the associated risk and required level of testing of the feed source. In general this is not an acceptable feed source as the likelihood of pathogen introduction is significant.
- Raw Offal/fish waste used as feed should not consist of aquatic animal species that are susceptible to any pathogens of concern to which the aquatic animals in quarantine are susceptible.
- Aquatic animal waste/offal should be sourced from negligible risk sources and testing of the waste/offal must occur for the pathogens to which the source, recipient, and local aquatic animal populations are susceptible.
- Freeze dried feeds – lyophilization preserves many pathogenic micro-organisms and thus requires the same risk assessment as offal or aquatic animal waste. For example, infective IPNv may be preserved for many years in freeze dried form depending on the storage medium (Wolf et al 1969).
Cross-Contamination Prevention
- Feeds must be protected from cross-contamination with other feeds or aquatic animal products prior to delivery at the quarantine unit in order to prevent pathogen introduction.
- Feeds must be obtained from a reputable source and stored in a manner that protects the feed from extremes of heat, light, and humidity at all times from manufacturing to feeding.
References:
Whipple and Rohovec. The effect of heat and low pH on selected viral and bacterial fish pathogens (report that IPNv will be killed with a process that reaches 65°C for 15 minutes followed by 5 minutes at 82°C). Aquaculture 1994;123:179-189.
Nygaard, H, Myrmel, N, Myrmel, M. Inactivation of fish pathogenic micro-organisms in fish by-products. Sub-Project IPN-virus. Nofima Report no. 30/2010. 2010; ISBN: 978-82-7251-801-0.
Wolf, K, Quimby, MC, Carlson, CP. Infectious pancreatic necrosis virus: lyophilization and subsequent stability at 4°C. Appl. Micr. 1969;17(4):623-624.
3.2.3.2 Feed Storage
Proper storage and handling of aquatic animal feed is essential to decrease predator and scavenger attraction to the premises. Feed to be used for multiple populations must be managed to prevent cross-contamination with feeds that are to be used in the quarantine unit.
Transportation vehicles should be cleaned and disinfected prior to picking up new feed supplies if the same vehicle is used for animal handling (live or dead).
Feed should be stored with a secure lid. Spilled feed, including excess oil leakage from feed bags, should be cleaned up immediately to decrease scavenger attraction to the quarantine unit. The feed storage area should be accessed daily in order to look for signs of access by scavengers/pests; if there are visible signs of scavengers/pests, preventive measures should be implemented such as checking that access points are blocked off, setting rodent traps, or calling the contract exterminator.
3.2.4 Fomites
3.2.4.1 Vehicle Management
Vehicles that contact aquatic animals, aquatic animal waste, equipment, or staff may become contaminated with pathogens of concern. Vehicle access to the quarantine unit must be limited to essential vehicles and vehicle parking areas must be clearly identified. Those vehicles that transport aquatic animals and/or enter the quarantine unit are subject to cleaning and disinfection procedures.
Water, detergent, and scrub brushes should be used to remove all visible organic matter from the outside surfaces of the vehicle prior to disinfection to ensure efficacy. Wheel wells and tires should not be missed. The cleaned vehicle should be rinsed with pathogen free water and allowed to dry before application of the disinfectant. The disinfectant should be applied and left in place for a duration as indicated on the manufacturer's instructions. The disinfectant should then be rinsed off with pathogen free water and left to dry.
Managing the quarantine unit equipment within the vehicle can remove the need for cleaning of the entire vehicle interior. Specific areas of the vehicle should be designated for quarantine unit materials to prevent cross contamination. Areas of the vehicle which are easily disinfected (e.g. trunk or box) should be used for transport of quarantine unit materials and all quarantine unit equipment should be placed into the designated quarantine unit area of the vehicle (e.g. in totes, in plastic bags etc.). Only the areas of the vehicle that have been in contact with the quarantine unit equipment and/or materials require cleaning and disinfection.
Disinfectants should be disposed of according to manufacturer's directions and meet the requirements of local waste management regulations.
3.2.4.2 Equipment Management
Equipment is to be kept clean and disinfected to limit the spread of pathogens of concern. Separate equipment must be designated for a single quarantine unit. All new equipment introduced to the quarantine unit must be cleaned and disinfected before use. Equipment should be designated for the quarantine unit and labelled with colour coding or name of the location. Cleaning and disinfection protocols must be followed for equipment that is transferred into and out of the quarantine unit. Acceptable disinfection methods for a quarantine unit include fallowing, drying, ultraviolet light, chemical disinfection or any other method approved by the CFIA. Products should be used according to the manufacturer's directions. Organic matter should be removed from equipment prior to disinfection to ensure efficacy. Disinfected equipment should be rinsed with pathogen-free water and allowed to dry.
Disinfectant concentrations should be maintained by checking concentration (test strips) or regular renewal of the product. Disinfectants should be disposed of according to manufacturer's directions and the requirements of waste management regulations.
3.2.4.3 Inorganic Waste Management – Disposal
Inorganic waste is material that is non-decomposable and does not consist of animal and/or plant matter.
Inorganic waste must be stored in secure containers and can be released from the quarantine unit without treatment once the aquatic animals are deemed to be of negligible risk for the pathogens of concern after the completion of disease freedom testing. Inorganic waste presents less risk of disease transfer out of the quarantine unit than organic waste. Inorganic waste disposal must be conducted in a manner that prevents the spread of the pathogens of concern outside of the quarantine unit. Several options exist for acceptable waste disposal including landfill (properly covered), incineration, and any other method that contains the waste from the aquatic animals on site and the surrounding environment. The quarantine unit must follow provincial and municipal requirements for waste disposal. Waste may be disposed of during the quarantine period upon approval by CFIA inspection if treated prior to disposal; the treatment system must be sufficient to destroy the pathogens of concern to which the aquatic animals are susceptible for the quarantine unit.
3.2.4.4 Cleaning and Disinfection of Equipment and Materials of the Quarantine Unit
The quarantine unit, animal holding units, and equipment must be constructed of materials that can be disinfected to ensure destruction of the pathogens of concern. Aquatic animal holding units and their plumbing located inside the quarantine unit must be constructed from materials that can be cleaned and disinfected. Preferred materials include plastics, fibreglass, and metals. Lower preference is porous materials (e.g. concrete).
Transportation materials and packaging must be made of materials that can be disinfected or made of disposable materials to ensure destruction of pathogens of concern. Aquatic animals and their products must be held in containers that are made from materials that can be cleaned and disinfected prior to entry and exit of the quarantine unit. Preferred materials include plastics, fibreglass, and metals. Lower preference is porous materials (e.g. concrete).
All equipment, packaging materials, vehicles or any other items which come in contact with the animals or water from the quarantine unit must be cleaned and disinfected in a manner which prevents the spread of pathogens of concern out of the quarantine unit. Several disinfection methods are acceptable including chemical disinfectants, physical disinfectants, or other methods approved by the CFIA that have been demonstrated as efficacious for destruction of the pathogens of concern. Chemical disinfectants must be labelled as effective for the destruction of the pathogens of concern and used according to manufacturer's directions. Physical disinfection procedures must reach the time and temperature profiles sufficient to destroy the pathogens of concern (e.g. steam cleaning). The entire quarantine unit must be cleaned and disinfected in a matter that is sufficient to destroy the pathogens of concern before the arrival of the aquatic animal population into the quarantine unit.
3.2.4.5 Chemical Storage
Chemical disinfectants must be stored in a manner that prevents their degradation and maintains efficacy. Manufacturer's instructions must be followed for proper storage of chemicals. The chemicals should be stored in a dry, secure area and in a location with the manufacturer's recommended room temperature. Expiry dates of chemicals should be noted before use and those expired should be properly discarded.
3.2.5 Vectors
3.2.5.1 Access Control
People, equipment or objects must not have access to the quarantine unit without the knowledge and control of the importer. Access to the quarantine unit must be prevented using a fence, locked door or another method that demonstrates that access is controlled. Signage must be visibly posted to indicate restricted access into the quarantine unit; the signage must be clear, legible and provide directions to the defined entry area or contact information for the office. The signage must be within the quarantine unit property boundary.
Anyone other than quarantine staff must contact the importer prior to entering the quarantine unit.
3.2.5.2 Quarantine Unit Biosecurity
Entry and exit procedures must be developed and implemented to prevent the introduction and spread of pathogens of concern on hands, feet, and clothing of all persons entering and exiting the quarantine unit. An ante-room is required as part of the entry and exit procedures. The goal of the ante-room is to provide a predefined transition area between the quarantine unit and the surrounding environment. The ante-room is not required to be a separate room from the quarantine unit and can be a designated area on the site or a clearly marked and segregated area within the quarantine unit. The ante-room area must be cleaned and disinfected regularly (at pre-defined intervals or upon accumulation of any organic matter). Site-specific clothing and footwear or the use of footbaths in entry and exit procedures of the quarantine unit is required and must be located in the ante-room. Disinfection stations must be provided and utilized in the ante-room and at all entry and exit points of the quarantine unit. Footbaths must be properly maintained, assessed daily for signs of fouling with organic material, and changed at regular intervals because they can become contaminated with micro-organisms and become a source of pathogen introduction. Only clean boots/shoes should be dipped in the footbath as footwear covered in organic matter will not be successfully disinfected. Where available, testing strips should be used daily to assess the footbath disinfectant concentration. Manufacturer's directions should be followed for the dilution of the disinfectant and staff provided with the container volumes and volumes of powdered concentrate or liquid disinfectant solution to add to a known volume of water (e.g. for a 1% solution, add 10 grams of powder per litre of water in the footbath).
It is preferred to have staff members work solely at the quarantine unit; however, if they must travel between the quarantine unit and other premises or rooms outside the quarantine unit, procedures must be implemented to prevent transfer of pathogens of concern on those staff members and their outerwear. Those wishing to enter the quarantine unit must be informed that they cannot have had contact with live aquatic animals or aquatic animals products, including wild aquatic animals, outside of the quarantine unit or been on another aquatic animal premises for 48 hours prior to visiting the quarantine unit.
A quarantine access logbook must be filled out by any persons wishing to enter the quarantine unit, which includes the attendance of previous aquatic animal locations within the past 48 hours. Quarantine staff must accompany anyone else who enters the quarantine unit at all times while on site and ensure they complete required the entry and exit procedures. Quarantine staff should inform those that enter the quarantine unit that they are not to handle aquatic animals, feed, and equipment. Access to sensitive areas (feed and mortality storage) should be restricted to quarantine staff unless access is required for inspection purposes. Refer to the example of a quarantine access logbook in Appendix C.
3.2.5.3 Predator, Scavenger, and Pest Control
Preventive measures and procedures must be in place to prevent access of predators, scavengers, and pests into the quarantine unit. The quarantine unit should use predator exclusion devices tailored to the specific needs of the site. Visible portions of the holding units should be checked, including the predator exclusion devices daily. Any damage to holding units or predator exclusion devices should be repaired as soon as possible. Feed should be stored and distributed in a manner that does not increase attraction to scavengers and predators and spilled feed should be cleaned up immediately. Waste must be properly stored prior to removal from the site and visible mortalities must be collected daily and stored securely and held separately from the aquatic animals in order to prevent access to predators, scavengers, and pests.
3.2.5.4 Mortality Management – Collection and Storage
The presence of mortalities can result in attraction of predators and scavengers and have negative effects on water quality and sanitation in the environment. Routine removal of mortalities minimizes pathogen spread. All visible dead and moribund aquatic animals must be removed on a daily basis and must be maintained in the quarantine unit in a biosecure location until disease freedom testing is completed and/or quarantine is released by CFIA Inspector. The dead animals must be identified, counted, and separated by container and/or unit. The containers used to collect and store mortalities must have secure, tight fitting lids that will exclude predators and scavengers, be leak-proof, and should be stored in an area away from the live aquatic animals. Moribund animals (animals exhibiting clinical signs or in a dying state) must be humanely euthanized prior to testing and/or disposal. Hygienic precautions should be taken when handling dead aquatic animals, which include wearing gloves while handling dead aquatic animals and washing hands after mortality removals.
The quarantine unit must have its own equipment that is associated with mortality collection, storage, and disposal and must be appropriately cleaned and disinfected according to manufacturer's instructions.
3.2.5.5 Mortality and/or Carcass Management – Disposal
Mortality and/or carcass disposal must be conducted in a sanitary manner to prevent the spread of the pathogens of concern to aquatic animals into or out of the quarantine unit. Various disposal methods are acceptable, including landfill (only if the animals are tested in quarantine and determined to be free of pathogens of concern), composting (only if the animals are tested in quarantine and determined to be free of pathogens of concern), autoclaving, ensiling, incineration, and any other method upon approval by the CFIA.
3.2.6 Specific Procedures for Active Observational Surveillance of the Quarantine Unit
Aquatic animals must be monitored and visually inspected daily by a trained individual who actively and systematically looks for diseased animals on a frequent daily basis while the animals are in quarantine. Moribund animals or mortalities must be collected daily and visually inspected by trained personnel for diseases of concern. Individual mortalities should be routinely examined internally for abnormalities or clinical signs of disease. The CFIA Inspector should be contacted when observations of clinical signs of disease occur. CFIA disease cards for specifics of diseases to which the quarantined species is considered susceptible should be available for staff
Procedures for active observational surveillance for aquatic animals:
- daily monitoring of behaviour (including feeding activity) and morbidity;
- daily mortality collection with specific instructions for when staff should contact management;
- routine, gross internal examination of mortalities; and
- emergency plan.
- Visually monitor for the following aquatic animal behaviours (if applicable):
- feeding;
- schooling;
- reaction to human presence;
- flashing;
- gasping; and
- visible, gross clinical signs of disease.
Mortalities should be classified and can include the following:
- systems-related (e.g. water quality);
- predator;
- handling/transport;
- clinical signs of disease—describe signs;
- fresh—freshly dead with no clinical signs of disease; and
- old—no longer suitable for diagnostic testing.
Specific triggers for the quarantine unit that indicate a serious disease occurrence:
- clinical signs of septicaemia (haemorrhages);
- ascites;
- trailing fecal casts;
- gray-coloured gills in live fish;
- presence of granulomas or other tissue lesions; and
- any other specific triggers for an individual site.
Tissue specimen collection for laboratory testing must be performed by CFIA inspectors.
Quarantine staff should be trained and knowledgeable of the biosecurity procedures and aquatic animal diseases to which the species are susceptible to prevent the introduction and release of pathogens of concern to and from the quarantine unit. The staff should be knowledgeable and experienced in monitoring aquatic animal health and the recognition of abnormal aquatic animal behaviour or clinical signs of disease. The quarantine unit should have an identified aquatic animal health professional or veterinarian associated with the quarantine unit who can be consulted during the quarantine period.
Appendix A: Example quarantine site diagram
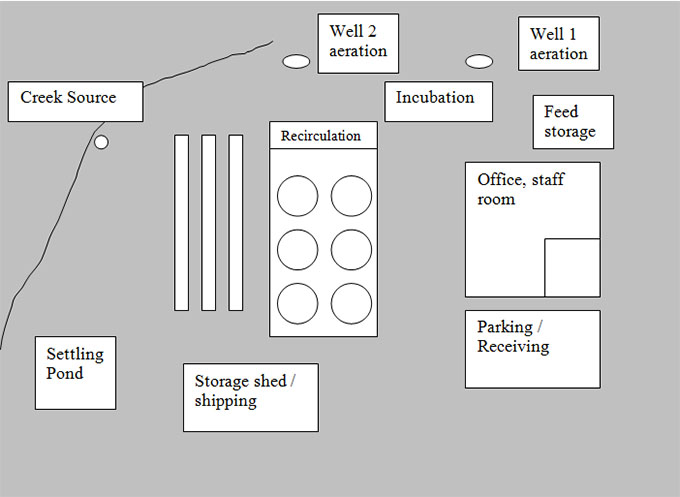
Description of quarantine site diagram - Example: 1. Add basic infrastructure
The quarantine site diagram shows a large rectangular area. Within this rectangular area, there are a number of boxes representing the placement of everything within the basic infrastructure of the quarantine site. At the far right corner there are two well aeration locations, with an incubation area below. Feed storage is located at the right side of the diagram, with the office/staff room and parking/receiving area below. The recirculation tanks are located in the middle of the diagram and the storage shed/shipping area is located below them. The settling pond is located at the far bottom left of the diagram to the right of the creek source, which is located at the far left of the site.
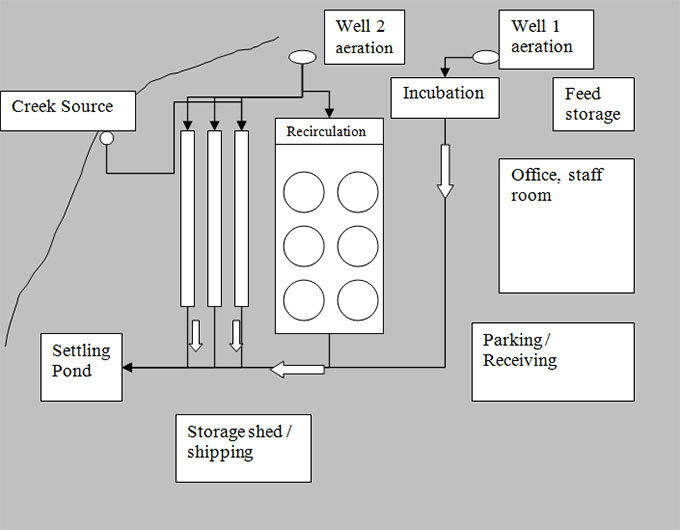
Description of quarantine site diagram - Example: 2. Superimpose water flow pattern
The quarantine site diagram shows a large rectangular area. Within this rectangular area, there are a number of boxes representing the placement of everything within the basic infrastructure of the quarantine site. At the far right corner there are two well aeration locations, with an incubation area below. Feed storage is located at the right side of the diagram, with the office/staff room and parking/receiving area below. The recirculation tanks are located in the middle of the diagram and the storage shed/shipping area is located below them. The settling pond is located at the far bottom left of the diagram to the right of the creek source, which is located at the far left of the site.
The water flow pattern is superimposed over the quarantine site diagram using arrows and lines.
Well 1 aeration supplies water to the incubation area. Well 2 aeration supplies water to the recirculation area and three separate tanks. The creek source also supplies water to the three separate tanks. The three effluent sources from the incubation, recirculation area and three tanks meet in a common water system, which then leads to the settling pond.
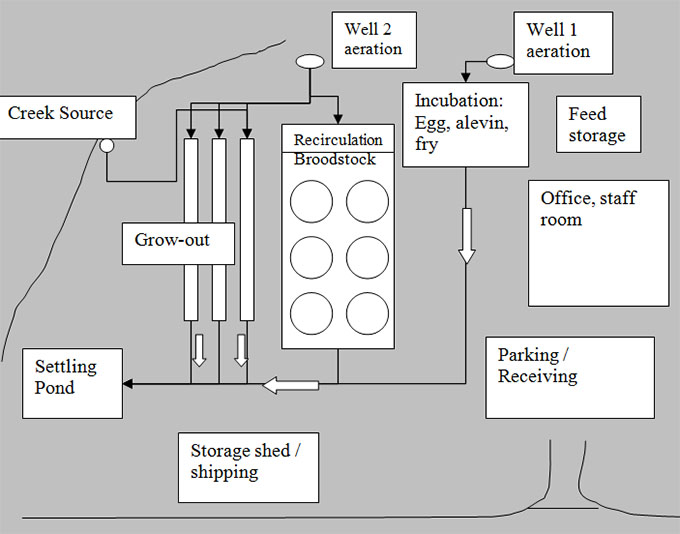
Description of quarantine site diagram - Example: 3. Add animal locations
The quarantine site diagram shows a large rectangular area. Within this rectangular area, there are a number of boxes representing the placement of everything within the basic infrastructure of the quarantine site. At the far right corner there are two well aeration locations, with an incubation area below. Feed storage is located at the right side of the diagram, with the office/staff room and parking/receiving area below. The recirculation tanks are located in the middle of the diagram and the storage shed/shipping area is located below them. The settling pond is located at the far bottom left of the diagram to the right of the creek source, which is located at the far left of the site.
The water flow pattern is superimposed over the quarantine site diagram using arrows and lines.
Well 1 aeration supplies water to the incubation area. Well 2 aeration supplies water to the recirculation area and three separate tanks. The creek source also supplies water to the three separate tanks. The three effluent sources from the incubation, recirculation area and three tanks meet in a common water system, which then leads to the settling pond.
The animal locations and gate/fence lines are superimposed over the quarantine site diagram using lines and labels. Eggs, alevin and fry will exist in the incubation area, broodstock will exist in the recirculation area, and grow out animals will exist in the three tanks. There are lines drawn at the bottom of the quarantine site diagram to represent fence lines and a gate that leads to the parking/receiving area.
Any locations that restrict the movement of people, animals or vehicle traffic can be further superimposed upon the diagram (e.g. gates, fence lines, etc.).
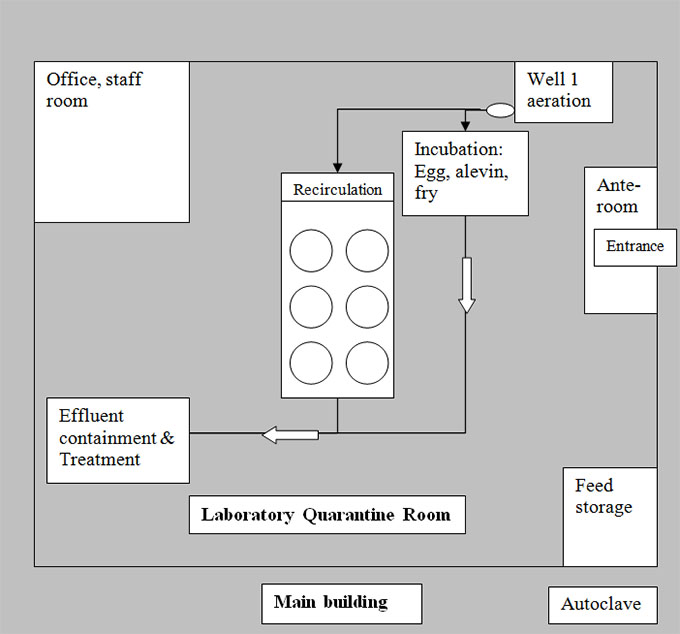
Description of quarantine site diagram - Example: 4. Laboratory Quarantine Room
The quarantine site diagram shows a large rectangular area. Within this rectangular area, there are a number of boxes representing the placement of everything within the basic infrastructure of the quarantine site. Lines and arrows represent water flow within the site diagram. A laboratory quarantine room is located within a main building. There is an autoclave located in the hallway of the main building. There is an entrance into the laboratory quarantine room within the main building that leads into an ante-room. There is a feed storage area at bottom right corner of the room and an office/staff room within the top left corner of the room. Well 1 aeration supplies water to the incubation area (where eggs, alevin and fry exist) and the recirculation area. The two effluent sources from the incubation and recirculation areas meet in a common water system, which then leads to the effluent containment and treatment area.
Appendix B: Example standard operating procedure for quarantine
This example of a generic procedure must be modified by the importer to the specifics of the quarantine unit, and their implementation will be verified during quarantine unit inspections.
This SOP is a generic example of the information that needs to be covered in a biosecurity plan. Each item that is italicized prompts the importer to enter site-specific information for the premises.
Water quality monitoring
Note: The maintenance of water quality is critical to good fish health management. However, this written procedure is limited to those water quality parameters that may impact the efficacy of water treatment and the disinfection of newly fertilized or eyed eggs and equipment. This information can be combined with the water treatment or egg disinfection SOPs, if desired.
Rationale: The efficacy of water treatment, equipment disinfection, and egg disinfection procedures are impacted by specific water quality parameters.
Definitions: List any definitions or acronyms required to understand the SOP.
Responsibility: Identify the staff names or positions that are responsible for the information in the SOP and for carrying out the procedure.
Required equipment: List all required equipment and locations; e.g. pH test strips, pH meter, refractometer, sample bottles, laboratory submission forms, reagents, glasswear, etc.
Procedure: The goal of this SOP is to ensure that water quality is monitored consistently and accurately, in order to be confident in the water quality parameters that impact water treatment, egg disinfection and equipment disinfection activities.
Acceptable parameters vary depending on the disinfection methods used for water treatment, egg disinfection, and equipment disinfection. Add the specific parameters that are commonly measured on your premises; e.g. turbidity prior to UV disinfection, pH prior to egg disinfection.
The water quality monitoring program should be designed to consider natural, seasonal water quality changes and the premises' specific needs. Describe the parameters measured routinely or those that change throughout the year due to changing conditions.
Water quality should be measured frequently enough to differentiate normal variation from declining water quality conditions. Clearly identify what level of each parameter is acceptable and the expected actions of staff if they encounter an unacceptable result.
pH is measured prior to egg disinfection and if chlorination/de-chlorination is used as a water treatment method.
Measure pH (insert frequency) using pH test strips/pH meter.
Describe calibration method for pH meter, or provide a web link or include a copy of the manufacturer's instructions.
Identify where in the system to collect the water sample.
Provide instructions for recirculation systems where the water quality is continually monitored on how to monitor the system, how to verify that chemical stores automatically added by the system are sufficient, and how to conduct calibrations.
Adjust pH to neutral using (insert chemicals or methods used to modify pH; e.g. sodium bicarbonate/calcium carbonate or flushing with fresh water to decrease un-ionized ammonia concentrations).
Provide the amount of chemical to add to a given volume of water, or calculations required for the specific chemical added.
Water quality parameters that may impact the efficacy of water disinfection are as follows:
- total dissolved solids;
- total suspended solids;
- iron manganese;
- hydrogen sulphide;
- calcium magnesium;
- residual ozone levels; and
- add any other parameters that need to be measured to ensure disinfection systems are effective.
Describe procedures used to monitor each of the above water quality parameters if they are a concern for your site, including frequency of testing. These parameters are measured after water filtration but before the water enters the UV unit. Identify the location in the water treatment system to collect a sample.
Water quality testing on site is conducted as follows:
- calibrate and maintain equipment (provide staff with manual); and
- identify levels of each water quality parameter that require action on the part of staff; e.g. TDS <800 to 1000 g/l; TSS <10 mg/l; Iron <0.3 mg/l; Manganese <0.05 mg/l; H2S—non-detectable; Ca/Mg <120 mg/l CaCO3.
Safety concerns: Provide staff with MSDS sheets for all chemicals used in water quality monitoring. Identify safety issues around use of UV (e.g. voltage) and ozone disinfection systems (risks of human exposure to ozone).
Contingency plan: Identify actions that staff is required to take in the event of deteriorating water quality.
Record-keeping requirements:
- identify records kept; e.g. fish health, results of diagnostic testing; and
- identify where records are stored.
Appendix C: Standard operating procedure guidelines for import quarantine
Note: The SOPs that are not applicable to dead aquatic animal carcasses or parts thereof are denoted with an asterisk (*).
This is table is not intended to be a complete list of requirements for a particular quarantine unit. Additional SOPs may be required after the CFIA has conducted the documentation and physical inspections.
Standard Operating Procedures
| Import Quarantine Procedures | Details |
|---|---|
| Unintentional animal introductions into the quarantine unit* | SOP:
Record-keeping:
|
| Intentional animal introductions into the quarantine unit | Record-keeping:
|
| Aquatic animal gametes and/or germplasm treatment* | SOP:
Record-keeping:
|
| Import Quarantine Procedures | Details |
|---|---|
| Unintentional introduction of water | SOP:
|
| Treatment and disposal of water and/or ice accompanying imported aquatic animals for quarantine | SOP:
Record-keeping:
|
| Assessment of influent water source* | Record-keeping:
|
| Water quality monitoring | SOP:
Contingency plan:
Record-keeping:
|
| Influent and effluent water treatment* | SOP:
Contingency plan:
Record-keeping:
|
| Disposal of solid waste collected from the quarantine unit | SOP:
Record-keeping:
|
| Import Quarantine Procedures | Details |
|---|---|
| Feed storage* | SOP:
Record-keeping:
|
| Import Quarantine Procedures | Details |
|---|---|
| Vehicle management | SOP:
Record-keeping:
|
| Equipment management | SOP:
Record-keeping:
|
| Inorganic waste disposal | SOP:
Record-keeping:
|
| Cleaning and disinfection | SOP:
Record-keeping:
|
| Chemical storage | SOP:
|
| Import Quarantine Procedures | Details |
|---|---|
| Access control | SOP:
Record-keeping:
|
| Quarantine unit biosecurity | SOP:
Record-keeping:
|
| Predator, scavenger, and pest control | SOP:
Record-keeping:
|
| Mortality management – collection and storage* | SOP:
|
| Mortality and/or carcass management – disposal | SOP:
Contingency plan:
Record-keeping:
|
| Import Quarantine Procedures | Details |
|---|---|
| Daily aquatic animal monitoring* | SOP:
Record-keeping:
|
| Visual examination of quarantine aquatic animal holding units for signs of disease* | SOP:
Contingency plan:
Record-keeping:
|
| Routine internal visual inspection of individual aquatic animal mortalities* | SOP:
Record-keeping:
|
| Staff training in biosecurity and aquatic animal diseases* | SOP:
|
Appendix D: Example quarantine access log
| Date YYYY/MM/DD |
Name and Phone Number | Company/Association | Last contact with aquatic animals or visit to another fish rearing facility | Time In | Time Out |
|---|---|---|---|---|---|
Appendix E: Susceptible Species
The CFIA Susceptible Species of Aquatic Animals table provides a list of the scientific names of susceptible species and identifies the disease(s) to which they are susceptible.
Susceptibility in aquatic species is determined if the infection can be demonstrated by natural occurrence of the disease in the species, or by experimental exposure of the species to the disease agent through a pathway that mimics a natural route of infection.
It is required to immediately contact the CFIA if reportable diseases are detected or suspected in the aquatic animals of the quarantine unit.