This document was part of CFIA's consultation on guidance for determining whether a plant is subject to Part V of the Seeds Regulations. This consultation ran from May 19, 2021 to September 16, 2021.
On this page
- 1. Introduction
- 2. Exemptions from Part V
- 3. Subject to Part V
- 4. Release of plants
- 5. Recourse mechanism
- Appendix 1 – Glossary of terms
- Appendix 2 – Environmental safety criteria
- Appendix 3 – Example scenarios
1. Introduction
1.1 Legislative authority
"Seed" is defined in the Seeds Act as "any part of any species belonging to the plant kingdom, represented, sold, or used to grow a plant." Part V of the Seeds Regulations, "Release of Seed", sets out the regulatory requirements for both the confined and unconfined environmental release of seed. Seed that is not substantially equivalent to seed of that species that is already present in Canada, in terms of its specific use and safety for the environment and human healthFootnote 1, is subject to Part V. Seed that is subject to Part V must be authorized before release into the environment.
1.2 Purpose and scope
Part V of the Seeds Regulations provides a mechanism to verify that the release of new plantsFootnote 2 does not have a negative impact on the Canadian environment or human health. This directive provides guidance to assist proponentsFootnote 3 in determining whether a plant is subject to Part V.
This guidance applies to all plants intended for release into the Canadian environment, including agricultural crops, horticultural plants, and forest trees, unless exempted under section 108 of the Seeds Regulations. Part V does not make distinctions between the various technologies that may be used in the development of a plant. For that reason, this guidance is focused on the plant and its interactions with the environment. A glossary of terms is provided in Appendix 1.
There are 2 possible outcomes when considering whether plant may be subject to Part V of the Seeds Regulations: either the plant is exempt from Part V, or the plant is subject to Part V (Figure 1).
Figure 1. Overview of possible outcomes under Part V of the Seeds Regulations.
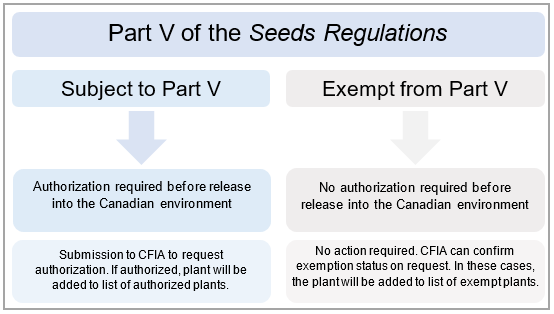
Description for Figure 1 – Overview of possible outcomes under Part V of the Seeds Regulations
Subject to Part V
If a plant is subject to Part V, the plant is regulated under Part V of the Seeds Regulations. A regulated plant requires an authorization from the Canadian Food Inspection Agency (CFIA) before release into the Canadian environment.
Further action is required:
- a submission must be made to CFIA requesting the authorization for release of the plant into the Canadian environment
- the plant will require an environmental safety assessment by the CFIA
The plant cannot be released into the Canadian environment before receiving an authorization by the CFIA. If authorized, the plant will be added to CFIA's list of authorized plants
Exempt from Part V
If a plant is considered exempt from Part V, no authorization from the CFIA is required. An exempt plant is eligible for release into the Canadian environment.
No further action is required:
- the plant does not require regulatory oversight under Part V of the Seeds Regulations
- the plant does not require an environmental safety assessment by the Canadian Food Inspection Agency
The plant may added to CFIA's list of exempt plants.
For the processes on obtaining an authorization to release seed into the environment, please refer to Directive 94-08 Assessment Criteria for Determining Environmental Safety of Plants With Novel Traits and Directive Dir 2000-07: Conducting Confined Research Field Trials of Plants with Novel Traits in Canada. These documents provide information about the submission, assessment, and decision process.
1.3 Responsibility to notify the Canadian Food Inspection Agency
It is the responsibility of the proponent to consider all the information provided in this document and to notify the Canadian Food Inspection Agency (CFIA) if their plant is subject to Part V of the Seeds Regulations prior to any release.
A proponent may request an opinion about whether a plant is subject to Part V from the CFIA at any stage of product development. For more information contact the Plant Biosafety Office (PBO) at PBO@inspection.gc.ca.
1.4 Privacy
Any information provided to the CFIA is subject to the Access to Information Act and the Privacy Act and will be protected in accordance with these acts. Information submitted to the CFIA may be made available to the public in accordance with these acts.
1.5 Other requirements
This directive does not address the importation of plants subject to Part V of the Seeds Regulations. More information is available in D-96-13: Import Requirements for Plants with Novel Traits, including Transgenic Plants and their Viable Plant Parts.
Plants intended for livestock feed and/or human food use may also be subject to requirements under the Feeds Act and Feeds Regulations and the Food and Drugs Act and Food and Drugs Regulations.
2. Exemptions from Part V
2.1 Statement on conventional breeding and gene editing
Virtually all plants developed by conventional breeding techniques qualify for an exemption from Part V, on the basis of being substantially equivalent to the lines they are derived from. Similarly, genetic changes that do not include foreign DNA will, for the most part, resemble conventional breeding outcomes, and will also qualify for an exemption. The CFIA recognizes that gene editing techniques can introduce genetic changes that are comparable to conventional breeding outcomes, and will also qualify for an exemption.
Plants derived from populations that have been previously grown in Canada qualify for an exemption, provided that they do not present new risks to the environment. Plants previously grown in Canada include those that were present prior to 1996 when Part V came into force, as well as those that were authorized after 1996.
2.2 Exemptions will increase as more products are authorized
Part V allows for subsequent plant lines to be exempted once an original event has been authorized in that species. Authorization decisions are listed on the CFIA's website. A plant will qualify for an exemption based on a past authorization if:
- the original authorization was issued without risk management conditionsFootnote 4
- the subsequent plantFootnote 5 does not contain foreign DNA
- the underlying mechanism of action is substantially equivalent to the original trait, and
- the trait does not result in one of the environmental impact outcomes listed in Section 3.3
Proponents can view the list of authorization decisions and exemption opinions as available. Proponents can use this information to identify if their plant is substantially equivalent and would qualify for an exemption from Part V. This exemption builds on the safety record of plant breeding, and allows for improved plant varieties to be continually developed.
3. Subject to Part V
If a plant is subject to Part V of the Seeds Regulations, an authorization must be obtained before the plant is released into the environment. There are 2 reasons for a plant to be subject to Part V. The plant is subject to Part V if it qualifies for one or more of the following:
- Newness
- Capacity to negatively impact the environment
Figure 2. Overview of decision-making.
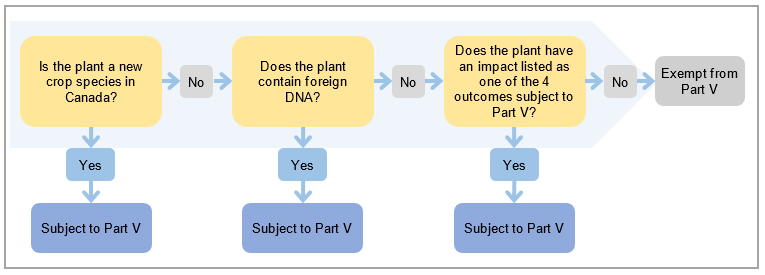
Description for Figure 2 – Overview of decision-making
There are 3 questions a proponent can ask themselves to determine if their plant is subject to regulation under Part V of the Seeds Regulations.
Is the plant a new crop species in Canada?
A plant species that has not been previously grown as a crop in Canada is subject to Part V.
Does the plant contain foreign DNA?
Foreign DNA commonly refers to DNA that is not currently a part of the organism's genome and is introduced through in vitro means. A plant that contains foreign DNA is subject to Part V.
Does the plant have the capacity to negatively impact the environment?
If the plant is not a new crop species in Canada and does not contain foreign DNA, the potential capacity of the plant to negatively impact the Canadian environment must be considered. The capacity of a plant to negatively impact the Canadian environment can be determined using 4 outcomes listed in Section 3.3. Plants that result in any of the 4 outcomes are considered to have a potential negative impact on the environment and are therefore subject to Part V.
Plants that are not a new crop species in Canada, do not contain foreign DNA and do not have the capacity to negatively impact the Canadian environment are exempt from Part V.
A proponent must identify whether the plant is a new crop species or includes foreign deoxyribonucleic acid (DNA). If so, then the plant is subject to Part V. However, if not a new crop and no foreign DNA has been incorporated into the plant, the proponent must then consider the plant's capacity to impact the environment. CFIA has identified 4 outcomes where a plant would have the capacity to impact the environment. Plants that result in one or more of these outcomes are subject to Part V. All other plants are exempt from Part V.
3.1 New crop species
A species that has not been previously grown as a crop in Canada is subject to Part V. Examples include:
- cultivation of a species previously found only in natural habitats
- cultivation of a domesticated plant species that has not been previously cultivated as a crop in Canada
3.2 Introduction of foreign DNA
A trait is new to a species when that trait is not derived from a pre-existing population of the species. The presence of foreign DNA is used to determine when a trait is new to a species. Foreign DNA commonly refers to DNA that is not currently a part of the organism's genome and is introduced through in vitro means. For example, a DNA sequence encoding a gene from a bacterial species is considered to be foreign DNA to the plant for which the sequence is introduced.
Regardless of the technique used to develop a plant, all plants that contain foreign DNA are subject to Part V.
For gene-edited plants, the DNA that encodes gene editing machinery (for example, CRISPR Cas protein(s) and associated guide RNAs) is considered to be foreign DNA. Plant developers often remove these sequences from the final lines by using plant breeding and selection. However, if a gene-edited plant still contains this foreign DNA within its genome, the plant is subject to Part V.
3.3 Capacity to negatively impact the environment
For plants that are not new and do not contain foreign DNA, the developer must consider whether the plant has the capacity to impact the environment.
When assessing if the release of a plant into the Canadian environment would have a negative impact on the environment or human health, the CFIA compares use and safety of the plant to its counterpart(s) already present in the Canadian environment. This comparative assessment is based on specific criteria outlined in Part V of the Seeds Regulations (for a description of how these criteria are applied, see Appendix 2). Based on the CFIA's experience with previous safety assessments, the CFIA has identified 4 plant breeding outcomes that have the capacity to negatively impact the environment.
- A trait that would make a plant more difficult to control by removing a management option
- A trait that introduces or enhances a toxin, allergen, or other compound that could reasonably be expected to have a negative impact on non-target organisms in the environment
- A trait that could reasonably be expected to improve the survival of plants in unmanaged ecosystems to such a degree that other species or ecotypes are displaced
- A trait that could reasonably be expected to result in the creation or enhancement of a plant pest or a reservoir for a plant pest
Plants that result in one or more of the 4 outcomes are subject to Part V. These 4 outcomes must be considered for both the plant of interest and any sexually compatible species that are present in Canada. Examples of these outcomes are provided in Appendix 3.
4. Release of plants
4.1 Confined release
A proponent may request authorization for a confined environmental release (research field trial) of a plant. This authorization is mandatory for all field trials of plants that are subject to Part V. However, electing to use the confined field trials program is not a definitive statement about whether the plant is subject to Part V. Confinement conditions will be lifted once a plant is authorized for unconfined release, or at any time if the plant is determined to be exempt from Part V. See Directive Dir 2000-07: Conducting Confined Research Field Trials of Plants with Novel Traits in Canada for information on how to apply for and conduct a confined research field trial.
4.2 Unconfined release
Further details about the process for receiving authorization for unconfined release are provided in Directive 94-08 Assessment Criteria for Determining Environmental Safety of Plants With Novel Traits.
Where an assessment concludes that a plant is equivalent to its counterparts, the CFIA will authorize the release without conditions. The authorization decision will be made publicly available on the CFIA website.
Where an assessment concludes that a plant poses a risk to environmental safety, the plant is considered to be a plant with a novel trait (PNT) and the CFIA may authorize the plant with conditions to manage the risk. Proponents can view the list of authorization decisions as available.
4.3 Release of plants that are exempt from Part V
If a plant is exempt from Part V, there is no requirement to submit information to the CFIA. The plant can be released in Canada, subject to any other applicable requirements (for example, authorizations for food and feed use, or variety registration).
On request, the CFIA will issue an exemption opinion letter. Proponents who would like an exemption opinion letter can contact the Plant Biosafety Office (PBO@inspection.gc.ca) to provide information about their plant and the rationale associated with their conclusions. The CFIA will review this information. If the CFIA agrees with the submitted rationale, the CFIA will issue a letter and will publish a summary of the exemption as available.
5. Recourse mechanism
CFIA will provide opinions to proponents on whether a plant is subject to Part V of the Seeds Regulations, and on whether the plant is a PNT. CFIA will also review submitted information, may request clarification or additional data prior to reaching a conclusion. The proponent may submit an appeal to the CFIA Complaints and Appeals Office if they believe that an incorrect conclusion has been reached.
Appendix 1 – Glossary of terms
- Conventional breeding
- breeding methods including the crossing and selection of plants, marker-assisted breeding, cell and embryo fusions, and chemical or radiation-based mutagenesis
- Mechanism of action
-
the biochemical process(es) through which genetic material determines a trait. This can include the specific DNA sequences, molecular targets, and biochemical interactions through which characteristics are achieved
Example 1: A specific amino acid in the binding pocket of an enzyme is altered, rendering the enzyme incapable of binding a herbicide, resulting in herbicide tolerance. Amino acid substitutions that affect the same binding pocket, and that result in the same insensitivity to the herbicide, are functionally equivalent mechanisms of action. In contrast, metabolizing the herbicide, sequestering the herbicide, or introducing an alternate enzyme would be different mechanisms of action.
Example 2: Functionally equivalent mechanisms of action can be achieved using many approaches. In one approach, RNAi technology is used to reduce the expression of an endogenous enzyme, interrupting flow through an endogenous metabolic pathway and resulting in a set of measureable traits. In an alternative approach, an edit to the endogenous promotor region is used to reduce the expression of the enzyme. The metabolic pathway is affected in the same manner. These mechanisms of action are equivalent, since they both reduce the expression of the target enzyme, leading to the same trait(s).
- Substantial equivalence
-
Plants are considered to be substantially equivalent to their counterpart(s) where there is no meaningful difference in the specific use and safety of the plant compared to plants of that species that have been grown in Canada. In considering whether a plant is substantially equivalent to its counterparts, consideration must be given to the plant's capacity to impact weediness, plant pest potential, impact on non-target organisms, impact on biodiversity, and the consequences of gene flow to sexually compatible species
Examples of substantial equivalence include:
- Using breeding techniques to combine existing desirable traits into a single line, provided there are no synergistic effects.
- Developing a trait where the same trait, with an equivalent mechanism of action, was either:
- present in a population of that species grown in Canada before Part V came into force in 1996, or
- assessed by CFIA and authorized without conditions in that species in Canada, or
- described in a CFIA opinion that states that the plant-trait-mechanism of action is exempt from Part V
- Trait(s)
- the characteristic(s) conferred to the recipient plant by specific genetic changes
Appendix 2 – Environmental safety criteria
This appendix provides additional details on how the environmental safety criteria are applied. These details are provided to explain how the CFIA considers whether a plant may have the capacity to have a negative impact on the environment. As well, if a proponent is uncertain whether their plant is subject to Part V of the Seeds Regulations, including whether the 4 outcomes identified in this guidance are applicable to their plant, it may be useful to consider the environmental safety criteria that are set out in Part V and explained below.
When the CFIA assesses the capacity of a plant to negatively impact the environment, the CFIA compares the plant to its counterpart(s) already present in the Canadian environment. As outlined in Part V of the Seeds Regulations, this environmental safety assessment is based on 5 criteria.
- Potential for the plant to become of weed of agriculture or be invasive of natural habitats
- the likelihood and persistence of herbicide-tolerant volunteers
- the likelihood that continued application of the same herbicide in subsequent rotations may increase selection pressure for herbicide-tolerant weeds
- Potential for and consequences of gene flow to sexually compatible plants
- the likelihood of gene flow from the plant to a related species, and whether gene flow may alter how the related species interacts with the environment
- Potential for the plant to become a plant pest
- the capacity of the trait to affect other pathways in the plant, including those involved in plant defence against pests
- the capacity of the trait to result in the creation or enhancement of a reservoir for a plant pest
- Potential impact of the plant or its gene products on non-target species, including humans
- the capacity for the gene product (for example RNA, protein) that confers the trait of interest to act as a toxin or allergen, and adversely impact non-target species, including humans as workers or bystanders
- Potential impact of the plant on biodiversity
- the risk of environmental impacts related to trait, which could include:
- a reduction in local plant biodiversity that adversely impacts other species in the food web
- persistence or spread of the plant outside its current cultivation areas that adversely impacts biodiversity in a natural, unmanaged environment
- field-evolved pest resistance to the trait, that would have downstream pest management consequences and related biodiversity impacts
- consequences of the release of the biomolecules into the environment (air, soil, water) during cultivation
- the risk of environmental impacts related to trait, which could include:
These 5 criteria serve as a framework that allows safety assessors to consider the potential for adverse impacts posed by a plant to the Canadian environment. This is accomplished through consideration of:
- characteristics of the plant, including ability to outcross to related species
- elaborated in species-specific biology documents published on the CFIA's website
- characteristics of the trait(s)
- phenotypic characteristic(s) conferred to the plant by specific genetic changes
- receiving environment
- the geographic distribution or cultivation area of the plant and related species in Canada
- interactions among a., b., and c.
The characteristics of the plant will dictate the applicability of the 5 key criteria. For example, the potential for and consequences of gene flow will only apply to plants with sexually compatible relatives in Canada. Thus, the potential for gene flow need not be considered for species such as corn and soybean, which do not have relatives in Canada. Similarly, if a plant species does not have weedy characteristics, there is no need to consider the potential for weediness or invasiveness unless there is a plausible risk hypothesis (for example, herbicide tolerance traits can increase selection pressure for herbicide tolerant weeds).
Appendix 3 – Example scenarios
The following examples are fictional, and are provided to illustrate how this guidance would apply in various scenarios.
Example 1: Exemption based on populations present before 1996
A single gene deletion is known to result in a flower colour change. Varieties of a plant species with this distinct flower colour have been grown in Canada since the 1950s. These plants are exempted from Part V under 108(a).
Using gene editing, a researcher interrupted the gene responsible for the flower colour change in a modern variety of this species. This produced the desired flower colour in a preferred agronomic background.
This plant qualifies for an exemption from Part V since:
- a plant population with this trait, in the same species, was present in Canada prior to 1996
- no foreign DNA has been introduced
- the underlying mechanism of action is substantially equivalent to the original trait
Example 2: Exemption based on an authorization issued after 1996
RNAi technology can be used to reduce the expression of an enzyme involved in fruit ripening. The CFIA has previously authorized a plant where delayed fruit ripening was achieved by using RNAi to reduce the expression of this ripening enzyme. An authorization was required in this case because RNAi technology involves the insertion of foreign DNA. A decision document on the CFIA's website outlines the CFIA's assessment and conclusions.
Using gene editing, a developer modified the promoter region of the same ripening enzyme in another variety of this plant. The expression of the enzyme was substantially reduced. As a result, the plant displays a delayed ripening trait that is comparable to the trait in the RNAi line that the CFIA has previously assessed and authorized.
This plant qualifies for an exemption from Part V since:
- this trait (delayed ripening) was previously authorized in this plant species by the CFIA
- no foreign DNA has been introduced in the edited plant
- the underlying mechanism of action (reduced enzyme expression) is substantially equivalent to the original trait
- the CFIA's previous assessment confirmed that this trait (delayed ripening) does not result in one of the environmental impact outcomes listed in Section 3.3
Example 3: Exemption based on no environmental impact – new or altered trait
Using gene editing, the sequence of a gene in a domestic crop was altered to resemble the orthologous gene sequence of a sexually compatible wild relative. The result was a plant with improved abiotic stress tolerance.
The improved plant produces more reliable yields, even under difficult growing conditions. The unmodified crop does not have inherently invasive characteristics, and this is also true of its wild relatives. The improved abiotic stress tolerance trait is an agronomic advantage, but is highly unlikely to make the improved plant or related species capable of displacing other species or ecotypes in a natural environment. No new toxins, allergens, or other compounds were introduced, and there is no indication or expectation that the trait would modify the interactions between the plant and pests and pathogens.
This plant qualifies for an exemption from Part V since:
- the plant is not a new species in Canada
- no foreign DNA has been introduced
- the trait does not result in one of the environmental impact outcomes listed in Section 3.3
Example 4: Subject to Part V – A trait that would make a plant more difficult to control by removing a management option
A proponent has developed a plant with herbicide tolerance by using mutagenesis and selection. Herbicide tolerance traits make a plant more difficult to control than its conventional susceptible counterparts, and the associated use of herbicides increases the selection pressure for herbicide tolerant weeds.
This plant is subject to Part V since:
- this trait must be considered under the plant breeding outcome in Section 3.3: a trait that would make a plant more difficult to control by removing a management option
The proponent must notify the CFIA and receive authorization prior to any release of seed. The proponent reviewed policies in Directive 94-08, and determined that herbicide tolerance is a familiar trait. This plant qualifies for a streamlined assessment tier.
Example 5: Subject to Part V – Foreign DNA and a trait that introduces or enhances a toxin, allergen, or other compound that could reasonably be expected to have a negative impact on non-target organisms in the environment
A proponent has developed a plant that expresses a bacterial gene. The bacterial gene encodes a protein with insecticidal characteristics.
This plant is subject to Part V since:
- foreign DNA was introduced (Section 3.2), and
- the insecticidal protein is a toxin to target insects, and could reasonably be expected to negatively impact non-target organisms, such as related insect species (Section 3.3)
The proponent must notify the CFIA and receive authorization prior to any release of seed. The proponent reviewed policies in Directive 94-08, and determined that this insecticidal protein is familiar. This plant qualifies for a streamlined assessment tier.
Example 6: Subject to Part V – A trait that could reasonably be expected to improve the survival of plants in unmanaged ecosystems to such a degree that other species or ecotypes are displaced
A proponent has improved cold stress tolerance in a plant, expanding the cultivation range of the crop in Canada. No foreign DNA was introduced. This plant species has a relative that is invasive of unmanaged ecosystems, and is capable of displacing native species. There has been documented gene flow between these two species in another country, so gene flow can reasonably be expected in Canada. Therefore, this cold stress tolerance trait could be transferred to the invasive relative via gene flow. If the invasive relative were to acquire this cold stress tolerance trait, it is reasonable to expect that this would improve the survival of the relative in unmanaged ecosystems where colder conditions prevail.
This plant is subject to Part V since:
- this plant and gene flow to its weedy relative must be considered under the plant breeding outcomes in Section 3.3: a trait that could reasonably be expected to improve the survival of plants in unmanaged ecosystems to such a degree that other species or ecotypes are displaced
The proponent must notify the CFIA and receive authorization prior to any release of seed. A pre-market safety assessment is needed to assess the impact of the cold stress tolerance trait on the Canadian environment through gene flow to the weedy relative.
Example 7: Subject to Part V – A trait that could reasonably be expected to result in the creation or enhancement of a plant pest or a reservoir for a plant pest
A proponent has developed a plant with altered secondary metabolites through non-selective mutagenesis. These plants are more palatable, but the trait is also linked to meaningfully increased susceptibility to fungal diseases under certain environmental conditions. The increased susceptibility occurs to a level that would require altered disease management strategies for effective management. If not adequately managed, this plant could represent a plant pest reservoir in comparison to other varieties of this plant species.
This plant is subject to Part V since:
- this plant must be considered under the plant breeding outcome in Section 3.3: a trait that could reasonably be expected to result in the creation or enhancement of a plant pest or a reservoir for a plant pest
The proponent must notify the CFIA and receive authorization prior to any release of seed.