This page has been archived
Information identified as archived is provided for reference, research or record-keeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
On this page
Mandatory use of digital equine infectious anemia test certificates in Canada
Summary
This document has been written for Equine Infectious Anemia (EIA) Accredited Veterinarians (AV) to address some key items raised in the feedback received via the Canadian Food Inspection Agency's (CFIA) online AV survey. The survey was open to all EIA AVs from February 8, 2021 to March 15, 2021 and 278 submissions were received. There were submissions from all provinces and territories where AVs practice, except Newfoundland and Labrador.
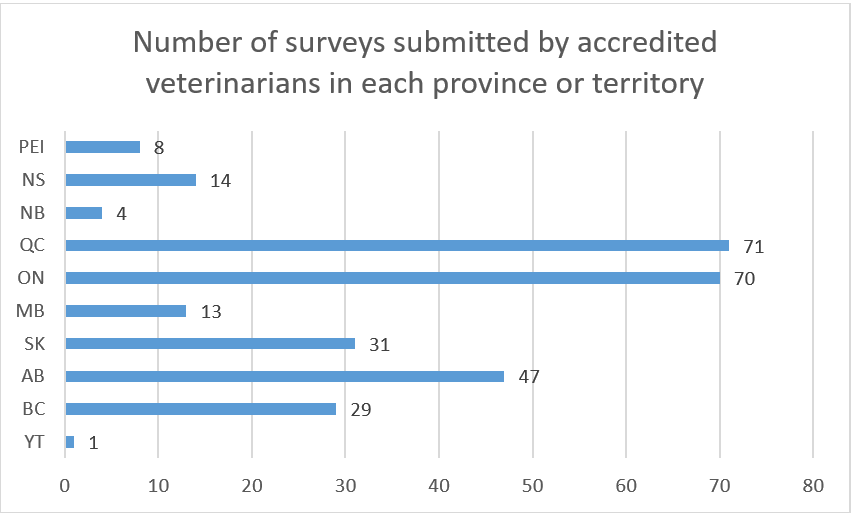
Description for number of surveys submitted by accredited veterinarians in each province or territory
The number of surveys submitted by accredited veterinarians in each province or territory:
- Prince Edward Island (PEI): 8
- Nova Scotia (NS): 14
- New Brunswick (NB): 4
- Quebec (QC): 71
- Ontario (ON): 70
- Manitoba (MB): 13
- Saskatchewan (SK): 31
- Alberta (AB): 47
- British Columbia (BC): 29
- Yukon (YT): 1
Equine infectious anemia AVs were asked to answer questions related to the CFIA's plan to make the use of digital EIA test certificates mandatory in Canada. As expected, a variety of opinions were expressed ranging from those who fully support the change to those who never want to go digital. Positive and constructive feedback was received in addition to some valid concerns. The following information is being provided to explain why the CFIA has decided to go in this direction, clarify some misconceptions about the digital system, and to provide an outline of next steps.
Themes
Going digital
The CFIA, and the federal government as a whole, have made it a priority to replace paper-based processes with digital ones that allow for online and real-time access to documentation. There are a variety of reasons for this including that data collection, data management, and having quick access to data can be extremely challenging for regulators when paper forms are used. In addition, the COVID pandemic has highlighted the need for processes that have fewer physical touch-points. The digital system also has better animal identification capabilities, has the potential for increased efficiencies for veterinarians, laboratories and regulators, and has been used successfully for years in the US and Canada. It is a system that has the capacity to continually and quickly evolve to meet the needs of its users.
Cost
There is a cost to use the digital system but this is often off-set by time-savings. Pricing is determined by the digital service provider and different options are available that are based on the amount of testing performed by a practice. Private practices and laboratories are responsible for setting their own fees based on business expenses and they should charge for their services accordingly. Some AVs may see a need to increase their EIA test fees when they start using the digital system but many others may not due to efficiencies realized when compared to the paper submission process. These efficiencies are likely to be more evident in subsequent years as opposed to when initial administrative steps are needed in the first year. All laboratories should realize significant in-house efficiencies when samples are submitted via the digital system.
Third party providers
The CFIA often uses third party providers, such as private AVs and private laboratories, to help deliver their animal health programs. Global VetLINK (GVL) is a third party technology provider that has a digital system that meets the CFIA's standards for functionality and security. If another company were to have a comparable system and they wished to engage in the review and approval process as GVL did, the CFIA would comply with the request. The CFIA's main role is as a national regulator and while the Government of Canada does develop and implement some technological tools themselves, they also work with external experts to provide Canadians with best available options.
Provenance
In today's world it is extremely common for Canadians to engage in day-to-day activities that involve businesses with their headquarters located in the US. These include companies such as Netflix, Costco, Amazon, Zoom, many credit card companies as well as those that provide practice management software, animal identification products, insurance and more. During the CFIA's review of the GVL system, emphasis was placed on making sure that it had been tested for several years, had a good reputation and provided users with the required functionality and security guarantees. Global VetLINK was not excluded from being approved for use in Canada because their headquarters is based in the US. The request from some AVs to pay GVL in Canadian funds has been brought to the company's attention.
Export certification
An EIA test certificate, whether in paper or digital form, is a supporting document that the AV and the CFIA (competent authority) use to confirm that the EIA-related import requirement of the receiving country has been met. The equine health certificate for export is a separate legal document issued or endorsed by the CFIA to attest compliance with the conditions of the importing country. The CFIA is currently rolling-out digital health certificates for the export of live animals to the US. Equines will be included in this initiative in the future and once this occurs, the digital export process will be even easier with the use of digital EIA test certificates.
Technical
Registration, training and ongoing support
Once GVL is contacted, the registration process can be completed within less than 1 business day, usually within a few hours. They provide easy to follow online training that can be done whenever it is convenient for the clinic and/or an individual staff member. Any veterinarian, laboratory personnel or animal owner that has a technical question about the system can contact GVL directly.
GVL Customer Success Team
7am – 7pm CST 7 days a week
Phone: 515-817-5704
Email: gvlsupport@globalvetlink.com
Website: Digital Equine Infectious Anemia (EIA) Tests (live chat, written & video resources)
Computer requirements
To use the GVL system, you must be able to access the internet and the recommended browsers are Google Chrome and Microsoft Edge. It is also recommended that veterinary staff use GVL's free HorseSync mobile app as a tool to help you easily upload equine photos and animal and client information while on location. There is no need to purchase new computer software to use the system because it is a web-based platform.
Storage capacity
Using the GVL system does not require a veterinary practice to store photographs on the practice server. This information is uploaded to, and stored on, GVL's server.
Taking photographs
Photographs do not need to be taken each time an equine is tested. They only need to be taken and uploaded onto the system the first time the animal's information is entered. When future testing is done, you just select the existing patient profile. Photograph requirements are outlined in module 8.4 of the Accredited Veterinarian's Manual. Getting good quality photographs is usually possible although sometimes it can be challenging due to the animal's temperament and/or the environmental conditions. It's recommended that when it's known that photographs need to be taken at an upcoming appointment, that practices notify clients of the need to have the animal adequately cleaned so that their markings are visible, as is necessary for the current paper form, and that they are located in an area with sufficient lighting. If it is known that an equine will not allow for proper photographs to be taken during the appointment, it's possible to ask the owner to take their own photographs, send them to the practice, and have the practice upload them onto the GVL system. The photographs must conform with the requirements listed in module 8.4 and you must be able to confirm that the submitted pictures match the animal you are sampling.
The CFIA is currently exploring the possibility of removing the requirement for photographs on digital EIA test certificates in very specific circumstances. See the microchip section below for more details.
Remote work locations
Global VetLINK's free HorseSync mobile app allows veterinarians and staff to enter client and equine information into the app when offline in remote locations. The created files will sync up with the GVL platform when the user is back online. GVL is also working to further expand their offline capabilities and plans to launch those later this year.
Consent
The CFIA will be updating some wording on the GVL digital test certificate which will allow for the removal of the current client informed consent process. This will simplify client registrations and result in additional time-savings for AVs. This change will come into force in the upcoming months and will be reflected on the GVL platform as well as in module 8.4 of the Accredited Veterinarian's Manual.
EIA approved laboratories
Regardless of whether a paper-based system or a digital system is used by an AV when submitting samples, all samples must be sent to a private Canadian laboratory that has been approved by the CFIA to perform EIA testing. All EIA approved laboratories in Canada use the GVL system and accept digital submissions.
Language
Digital and paper EIA test certificates include both official languages. The Canadian GVL platform has the ability to detect the language of the user's internet browser and respond accordingly. For example, if you are working in French, the French version of the platform appears. Also, if you use a French browser when accessing GVL training videos, and enable the closed caption [CC] function, subtitles will be in French. Global VetLINK can provide technical support in both official languages when the online chat function is used.
Implementation timing
The majority of respondents were either supportive of, or neutral about, the implementation of the mandatory use of digital EIA test certificates by the fall of 2021. Some expressed concerns that an implementation date in early fall did not provide everyone with enough time to register and complete the online training so this has been taken into consideration. See Next steps section for details.
Exemption for low-volume testers
Practices, not individual veterinarians, that do 10 or less EIA tests per calendar year have the option to apply for an exemption from having to use the digital system. Those who are granted an exemption will be permitted to submit samples for testing using the paper Form CFIA/ACIA 3937, Equine Infectious Anemia (EIA) Serum Test Report and Certificate. Approved laboratories will not be permitted to perform testing on samples submitted using paper Form CFIA/ACIA 3937 unless they have been notified by the CFIA in advance that the practice has been approved for an exemption. Instructions on how to apply for the exemption will be communicated to AVs later this year and made available in module 8.4 of the Accredited Veterinarian's Manual.
Multi-horse testing form
As of December 1, 2021, paper Form CFIA/ACIA 4679, Equine Infectious Anemia (EIA) Domestic Serum Test Report and Certificate will no longer be accepted for EIA sample submission. The digital system is well suited for multi-horse clients and multiple submissions can be performed efficiently with it.
Microchip
The CFIA is exploring the possibility of removing the need for equine photographs when creating a digital EIA test certificate if the animal is microchipped with a unique number that can be verified with a chip reader at the time of sampling and the testing is only needed for domestic purposes and not for export. The CFIA will update AVs on this development when more information is available.
Next steps
Accredited veterinarians will be required to use the digital EIA test certification system starting December 1, 2021.
Already registered
You don't need to do anything else but if there are some employees in your clinic that aren't familiar with the system yet, have them reach out to GVL to get the support they need to get comfortable with using it.
Not registered
Contact GVL so they can help your clinic get set up and staff comfortable before December 1, 2021.
Apply for an exemption
If your practice meets the low-volume testing exemption criteria of 10 or less tests per year, you will have the option to apply for an exemption from having to use the digital EIA test certification system. Later this year the CFIA will send out information about the exemption application process. Applicants will need to submit their request before the due date to ensure that their submissions can continue to be done using the individual paper Form CFIA/ACIA 3937.
The CFIA will notify national equine industry organizations and provincial and territorial contacts of the upcoming transition and the following points will be highlighted.
- The CFIA is making the use of digital EIA test certificates mandatory in Canada as of December 1, 2021. The current paper version of the individual EIA test certificate will only be allowed in unique circumstances.
- The actual EIA test has not changed but there are a few differences between the paper and the digital certificates. What owners will notice is:
- the digital certificate includes photographs of the equine instead of hand drawings
- EIA test certificates can be accessed online in real-time
- EIA test certificates can be printed off when a hard copy is needed
- A client's veterinarian will need to take a few pictures of the equine the first time they are tested using the digital system. To ensure good quality photographs can be obtained, it will be important for owners to make sure the equine is clean enough so that their markings are visible. They also must be located in a well-lit area. Once the pictures are in the system they don't need to be done again and future testing will be very simple for everyone.